Steel is one of the most sought after materials in the world today. Without it, it is difficult to imagine any existing construction site, engineering enterprises, and many other places and things that surround us in everyday life. At the same time, this alloy of iron and carbon can be quite different, therefore this article will consider the influence of alloying elements on the properties of steel, as well as its types, grades and purpose.
Classification by purpose
Each steel, depending on what it is created for, can necessarily be classified into one of the following categories:
- Structural.
- Instrumental.
- Special purpose with special properties.
The most numerous class is structural steels, designed to create a variety of building structures, instruments, and machines. Structural grades are divided into upgradeable, cemented, spring-spring, and high-strength.
Tool steels are differentiated depending on the tool for which they are produced: cutting, measuring, etc. It goes without saying that the influence of alloying elements on the properties of steel in this group is also great.
Special steels have their own division, which includes the following groups:
- Stainless (aka corrosion-resistant).
- Heat resistant.
- Heat resistant.
- Electrical.
Corrosion-resistant (stainless) steels and alloys
BASICS OF THE THEORY OF CORROSION
The destruction of metals and alloys as a result of chemical or electrochemical action on their surface of an external aggressive environment is called corrosion .
Corrosion, as a rule, is accompanied by the formation of corrosion destruction products on the metal surface. For example, on the surface of iron alloys, as a result of corrosion, rust is formed, which has a brown color. In some individual cases, metal corrosion is not accompanied by the formation of such noticeable destruction products, and then its appearance is quite difficult to detect.
Corrosive destruction is the result of the interaction of a metal with the external environment and the intensity of its development depends on the properties of the metal itself, as well as on the nature of the environment. Most metals, being resistant in some environments, are quite easily destroyed when interacting with other environments. For example, copper alloys are stable in humid atmospheres, but are highly susceptible to corrosion if even small amounts of ammonia are present in the atmosphere; Tantalum and titanium at room temperatures are very stable in many aggressive environments, but they acquire high chemical activity when heated above 600 ° C.
There are several types of corrosion: continuous or uniform, when the entire surface of the product is exposed to corrosion; point or local, if corrosion develops in individual small areas; intergranular corrosion (ICC), when corrosion spreads deep into the product along the grain boundaries; Stress corrosion is the occurrence of corrosion cracks due to the simultaneous impact of tensile stresses and an aggressive environment on the metal.
Corrosion can occur as a result of purely chemical reactions with the environment, as well as due to electrochemical processes occurring at the interface between the metal and the external environment. The largest amount of metal is destroyed as a result of electrochemical corrosion.
Electrochemical corrosion is the destruction of metals and alloys when exposed to electrolytes. This type of corrosion is characterized by the flow of electric current, the transition of atoms to an ionized state and other electrochemical processes.
The most common electrolytes in practice are aqueous solutions of salts, acids and alkalis. Thus, electrochemical corrosion includes corrosion of metal containers, pipelines, machine parts and parts of stationary structures under the influence of acids, sea, river, ground and other waters. The most common is atmospheric corrosion.
If there are two metals with different electrode potentials in the electrolyte, then the metal with a more negative electrode potential (anode) continuously releases ions into the solution (dissolves), and the resulting excess electrons continuously flow into the metal with a less negative electrode potential (cathode). The cathode in the contact pair is not destroyed; electrons from it are continuously removed into the external environment.
All metals can be arranged in a row in descending order of their electrochemical potential:
Metal…………………. Au Ag С u Η Ni Fe Ζ n Α l
Electrode potential, V +1,42 +0,80 +0,34 0 -0,23 -0,44 -0,76 -1,66
In technical metals and alloys, which are polycrystalline bodies, the microstructure consists of grains of one or more phases, non-metallic inclusions, etc. These different structural components, which have different physical and chemical properties, upon contact with the electrolyte acquire electrode potentials of unequal magnitude and sign and some of them will become anodes, and others - cathodes. Thus, industrial metals and alloys, when exposed to electrolytes, can be considered as multielectrode elements consisting of a huge number of microscopically small corrosive galvanic pairs - microgalvanic pairs. The more the electrode potentials of the phases in the alloy differ, the faster its corrosion destruction occurs (in particular, dendritic segregation is precisely why the resistance to electrochemical corrosion decreases). It follows that either very pure metals or alloys that have a uniform (homogeneous) solid solution structure can have high corrosion resistance.
The passive state is the state of a metal (alloy) when it exhibits increased corrosion resistance (even practically stops corroding) in an aggressive environment. The opposite state, when the same metal corrodes, is called the active state.
Experimental data show that the transition of a metal from an active to a passive state is associated with an increase in its potential. For example, iron in its normal state has an electrode potential of -0.4 V; in a passive state, its potential can rise to +1.0 V.
Effect of doping. There are two groups of corrosion-resistant metals. Some metals resist corrosion well due to their low chemical reactivity. Others, being by their nature active elements, acquire high chemical stability due to the phenomenon of passivity. The first group includes platinum, palladium, gold, the second group includes chromium, titanium, aluminum, etc. To increase the corrosion resistance of a reactive metal, alloying elements are introduced into it.
When a metal is alloyed with another, more noble metal, the potential of the alloy initially remains virtually unchanged. But when a certain concentration is reached, a jump in potential occurs and the corrosion resistance of the alloy in a given environment also increases abruptly, and boundaries (thresholds) of stability appear.
It was experimentally established that such sharp changes in stability occur when the ratio of atoms of the alloying element to the alloyed element is a multiple of 8, i.e. n/8, where n is an integer 1, 2, 3... This corresponds to 12.5; 25; 37.5...% (at.) alloying element.
The appearance of stability boundaries is explained by the fact that when the alloy interacts with an aggressive environment, some of the atoms of the base metal go into solution, and the remaining atoms of a more noble or easily passivating metal form a kind of barrier on the metal surface. This barrier consists either of the noble metal atoms themselves, or of protective shielding films.
In more active environments, a higher concentration of a stable element is required, i.e. in this case, the limits of stability arise at a higher value of the number n.
The stability limit is also observed in alloys in which one of the components has the ability to self-passivate. This boundary is also observed in systems when one of the components in a given aggressive environment forms protective shielding films from insoluble compounds.
The corrosion resistance of steel can be increased if, firstly, the carbon content is reduced to the minimum possible amount and, secondly, an alloying element that forms solid solutions with iron is introduced in such an amount that the electrode potential of the alloy increases abruptly.
Steel that is resistant to atmospheric corrosion is called stainless steel. A steel or alloy that has high resistance to the corrosive effects of acids, salts, alkalis and other aggressive environments is called acid-resistant.
CHROME STAINLESS STEEL
Chromium is the main alloying element that makes steel corrosion resistant in oxidizing environments. The corrosion resistance of chromium stainless steels is explained by the formation of a protective dense passive film of Cr2O3 oxide on the surface. Such a film is formed only when the chromium content is more than 12.5% (at.). It is at this chromium content (n=1) that the potential changes abruptly from -0.6 to +0.2 V.
Iron and chromium form a continuous series of solid solutions (See iron - chromium diagram). Thanks to this, it is possible to obtain steel with a high chromium content in solid solution. Chromium is not a scarce metal, its cost is relatively low, so chromium steels are the cheapest stainless steels. These steels have a fairly good set of technological properties. Carbon in stainless steels, including chromium steels, is an undesirable element, since, by binding chromium into carbides, it thereby depletes the solid solution of chromium, reducing the corrosion properties of the steel. In addition, carbon expands the region of the γ-solid solution, facilitating the formation of a two-phase state (Fig. 1).
Rice. 1. The influence of carbon on the position of the γ region - solid solution on the iron - chromium diagram
With a higher chromium content, the σ phase will be present in the steel.
The higher the chromium content, the higher the corrosion resistance of chromium steels. Currently, three types of chromium steel are smelted: 1) containing 13% Cr; 2) 17% Cr- 3) 25-28% Cr.
Steels 08X13 and 12X13 have increased ductility and are used for the manufacture of parts subject to shock loads (turbine blades, cracking unit fittings, household items, etc.).
From steels 30X13 and 40X13, which acquire a martensite structure after heat treatment, measuring and medical instruments, springs and other corrosion-resistant parts that require high hardness or strength are made.
Steels containing 17 and 25-28% Cr are classified as ferritic steels. They have higher corrosion resistance compared to X13 steels. When heated above 850° C, ferritic steels tend to grow grains and their ductility decreases. To obtain a single-phase structure and reduce the tendency to grain growth and MCC, titanium and niobium (08X17T, 15X25T) are added to these steels. Strength increases, ductility remains sufficient, and the properties of welds improve. These steels are used for the manufacture of equipment operating in such aggressive environments as fuming nitric acid, phosphoric acid, and make equipment in the chemical and food industries corrosion-resistant. Heat exchangers for hot nitrous gases, pipelines and tanks for acids, etc. are made from 12X17 steel.
The introduction of molybdenum (12Х17М2Т) makes the steel resistant even to organic acids (acetic, formic). Ferritic grade steels are not susceptible to stress corrosion.
For the manufacture of ball bearings operating in aggressive environments, steel 95X18 (0.9-1.0% C, 17-19% Cr) is used.
All chromium steels are subjected to hardening at 1000-1100°C followed by tempering (for ferritic steels - at 700-750°C, martensitic class 200-250°C).
Ferritic steels do not undergo transformations when heated, so heat treatment is carried out to obtain a more homogeneous solid solution structure, which increases corrosion resistance.
CHROME-NICKEL STAINLESS STEEL
Nickel is one of the metals that easily acquire passivity, although its passivation ability is less than chromium and molybdenum. Adding nickel to iron in an amount of 1/8 mole dramatically improves the corrosion resistance of the alloy in sulfuric acid. At a nickel concentration of 2/8 mole, the corrosion resistance increases even more.
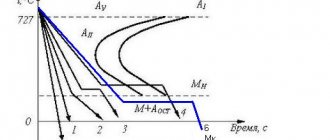
Iron-nickel phase diagram. Nickel is an austenite-forming element that greatly reduces the critical points of the γ-να transformation. Nickel also has this effect when it is introduced into chromium steels. Therefore, steel containing 18% Cr and 9% Ni has an austenite structure at room temperatures (see Fig. 2).
Rice. 2. Structural diagram of stainless steels
Stainless steels with an austenitic structure have higher corrosion resistance,
better technological properties compared to chromium stainless steels, in particular, better weldability. They retain strength to higher temperatures, are less prone to grain growth when heated, and at the same time, austenitic steels do not lose ductility at low temperatures. Like chromium steels, chromium-nickel steels are corrosion-resistant in oxidizing environments. The main element that increases the potential of iron is also chromium, so its content should be >13%. Nickel only further increases the corrosion resistance of steels.
The composition and properties of chromium-nickel stainless steels are given in GOST 5632-72. In Fig. Figure 2 shows a structural diagram that allows you to determine the structure of steel depending on its composition.
Chromium-nickel steels, depending on composition and structure, are divided into steels of austenitic, austenitic-martensitic and austenitic-ferritic classes.
The lower the carbon content, the higher the corrosion properties of stainless steels. The carbon contained in chromium-nickel steels can be in solid solution, as well as in carbides or carbonitrides of varying degrees of dispersion. Cr23C6 carbides are predominantly formed, and they are formed already at a carbon content of slightly more than 0.04% (0.04% C is the solubility limit of carbon in austenite alloyed with nickel). If steels contain nitrogen (for example, X17AG14 steel), then carbonitrides of the Me23(C,N)6 and Me(C,N) type can be formed.
Most chromium-nickel stainless steels belong to the austenitic class: 04Х18Н10, 12Х18Н9Т, 09Х14Н16Б, 08Х10Н20Т2, etc. These steels are ductile, easy to weld, have increased heat resistance, and are corrosion-resistant in many environments with moderate activity. Steel 12Х18Н10Т is the cheapest and therefore most often used.
For greater homogeneity, chromium-nickel steels are quenched at 1050-1100 ° C in water. In this case, σΒ = 50-60 kgf/mm2 and δ = 35-45% are obtained. These steels are strengthened by cold plastic deformation.
Additional alloying of chromium-nickel steels with molybdenum and copper increases their corrosion resistance and acid resistance (03Х16Н15МЗ, 03Х17Н14М2). Sometimes titanium and aluminum are introduced into these steels in small quantities, which, forming dispersed intermetallic compounds of the Ni3(Ti,Al) type, strengthen the austenite (08Х17Н13М2Т, 08Х17Н15МЗТ).
Steel 06ХН28МДТ (0.06% C; 22-25% Cr; 26-29% Ni; 2.5-3% Mo; 2.5-3.5% Cu and 0.5-0.3% Ti) has high corrosion resistance, it is used in highly aggressive environments (diluted sulfuric acid, etc.). This steel, after quenching at 1100″C in water, has an austenite structure with a small amount of carbonitrides. After short-term heating to 500-900° C, it does not show a tendency to MCC.
Nickel is a fairly expensive and scarce metal, so stainless steels are created with a lower nickel content. To do this, other austenite-forming elements are introduced into the composition of stainless steels, for example, manganese and even nitrogen (steels 10Х14Г14Н4Т, 15Х17AG14, 10Х14AG15, etc.).
Austenitic-martensitic steels (transitional steels) have lower corrosion resistance compared to austenitic steels, but exceed them in strength (σΒ = 120-130 kgf/mm2). Transitional class steels include steels 09X15N8Yu, 09X17N7Yu, 08X17N5MZ, 20X13N4G9, etc.
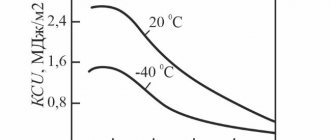
The heat treatment regime of these steels is characterized by great complexity: hardening, cold treatment, tempering - aging. In Fig. Figure 3 shows the effect of various types of heat treatment on the strength of stainless steels of various classes. Transitional class steels receive the greatest strengthening. Such steels are used to create lightweight structures with high resistance to corrosion damage.
Rice. 3. The influence of heat treatment on the strength of stainless steels:
1 - hardening; 2 - hardening and cold treatment; 3- hardening, cold treatment, tempering (aging)
Austenitic-ferritic steels are proposed as substitutes for chromium-nickel steels of the X18N8 type in order to save nickel. This class includes steels 12Х21Н5Т and 08Х22Н6Т. Austenitic-ferritic steels at room temperatures have higher strength and hardness than steel type 18-8, but their ductility and toughness are lower. These steels do not have stable properties: their properties depend on the ratio of ferrite and austenite phases, which in turn depends on the total influence of ferrite-forming (Cr, Ti, Mo, Si) and austenite-forming (Ni, N2, C) elements. With an increase in the amount of ferrite, the heat resistance of steels decreases, the strength increases, and the ductility decreases, but not below 30%. Good technological properties are obtained with the ratio Φ:A=1:1.
Steel 15Kh28AN, which has good mechanical properties, also belongs to this class of steels.
(σΒ = 65–70 kgf/mm2, δ = 11–23%), including in the weld.
Typical heat treatment of austenitic-ferritic steels: hardening from 1000-1150° C and tempering - aging at 500-750° C.
Austenitic-ferritic steels are not subject to stress corrosion cracking: cracks can only occur in the austenitic areas, but the ferritic areas retard their development.
Stainless steels exhibit a special type of corrosion called intergranular corrosion (sometimes also called intergranular corrosion). Such corrosion occurs mainly along grain boundaries and is very dangerous because it does not have any external signs - the metal even retains a metallic luster. At the same time, the strength drops catastrophically, the metallic sound disappears, the metal is so easily destroyed that it can be turned into powder. Intercrystalline corrosion (ICC) develops if a stainless steel product, after hardening, is heated to 500–700° C or if slow cooling is carried out in this temperature range. At the same time, a network of chromium carbides is clearly visible in electron micrographs.
The causes of ICC have been studied for many years and there are several theories explaining the causes of this dangerous phenomenon.
The most accepted is the so-called “impoverishment theory.” It is known that the grain boundary is a transition zone between them.
If the penetration of a dissolved impurity into the intergranular zone reduces the excess energy of the boundaries, the concentration of this impurity in the zone increases. It has been established that carbon reduces the excess energy of boundaries, therefore intercrystalline internal adsorption of carbon occurs along the grain boundaries of stainless steel. Thus, already during quenching, carbon atoms are nonuniformly distributed in the solid solution, their concentration along the boundaries is greater than in the grain. Although chromium carbides are not formed in this case, such an increased concentration of carbon is, as it were, a preparation for their rapid formation. When heated to 500-700° C, chromium carbides Cr23C6 are formed along the grain boundaries. At these temperatures, the diffusion of carbon in solid solution to grain boundaries proceeds faster than that of chromium. Therefore, the formation of carbides consumes not only the carbon reserve present there, but also the carbon diffusing from inside the grains. At the same time, chromium, necessary for the formation of carbides, comes, at the first stages of the process, from the boundaries or from the boundary zones of austenite. As a result, the chromium content in the border zones of grains becomes less than 13% (even up to 6.5%) and they lose their corrosion resistance.
Due to the great danger of the MCC phenomenon, all smelted stainless steels must be checked for susceptibility to this type of corrosion. In this case, samples made of hardened steel are subjected to provocative tempering for an hour at 650° C. After this, the samples are boiled in an aggressive environment and the presence of MCC is determined.
The tendency to MCC in stainless steels can be eliminated: 1) by reducing the carbon content (in steels containing 0.02% C, MCC is not observed); 2) the introduction of elements - titanium or niobium stabilizers, which have a greater affinity for carbon than chromium; 3) using stabilizing annealing (heating the product to 850°C).
Rice. 4. Microstructure of chromium-nickel stainless steel 08Х18Н9 without MCC (a) and with MCC. (b)
When welding in the heat-affected zone, the metal can heat up to dangerous temperatures (500-700 ° C). Therefore, if the steel is prone to MCC, then welded products should not be made from it, or after welding it is necessary to carry out heat treatment, at least annealing to 650°C. In Fig. Figure 4, α shows the microstructure of stainless steel 08Х18Н9 after heat treatment (quenching at 1100° C in water), heating at 650° for an hour and boiling in sulfuric acid for 48 hours (Fig. 4, b).
CORROSION-RESISTANT ALLOYS AND CAST IRONS
In addition to stainless steels, other corrosion-resistant alloys are also used in industry.
For particularly aggressive environments, nickel-based alloys such as Hastelloy (NIMO alloys) are used. The nickel content in these alloys reaches 80%. The second element present in these alloys in large quantities is molybdenum (15-30%). The composition of some alloys is given in table. 1.
Table 1.
Chemical composition (%) of acid-resistant nickel-based alloys such as Hastelloy
| Alloy | WITH | Μn | Si | Cr | Mo | Fe | Other elements |
| Hastelloy A (EI460) | <0,12 | <3 | <1 | _ | 20 — 22 | 18 — 20 | |
| Hastelloy V (EI461) | <0,12 | <3 | <1 | <1 | 26 — 30 | 4 — 7 | 0.3V |
| Hastelloy S (EP375) | <0,12 | <1 | «1 | 15,5— 17,5 | 16 — 17 | 4,5 -7 | 3.75-5.25W |
| Hastelloy D | <0,12 | 0,8 -1,25 | 8,5-10 | <1 | _ | <1 | 3.6-6.5Cu |
These alloys have very high corrosion resistance in environments where, apart from them, only a few metals are stable (for example, in boiling phosphoric acid up to a concentration of 50%, in boiling hydrochloric acid up to 20%, etc.).
Hastelloy alloys have high mechanical properties, which can be improved by heat treatment - hardening + aging at 800° C. In this case, σв = 120 kgf/mm2 and hardness ΗВ = 360.
The disadvantage of alloys is their tendency to MCC, so the carbon content in them should be minimal.
Corrosion-resistant cast irons are resistant to many aggressive environments (and not only oxidizing ones). They are usually heat resistant. Alloy cast irons are cheaper than stainless steels and have good casting properties, so products made from them are produced by casting methods. The chemical composition and properties of acid-resistant cast iron are given in GOST 2176 and GOST 2233 (Table 2).
Chromium cast irons contain 26-36% Cr. The structure of chromium cast iron is a solid solution of chromium ferrite and eutectic carbides. Carbides can also be in a free state, and Cr7C3 carbides are predominantly formed. Chromium cast irons (X34) have high hardness (HB 325-400), resist wear well, but are difficult to machine. Alloys 25X18L and 30X20L are related to steel in terms of carbon content, and cast iron in terms of properties. Their casting and mechanical properties are better than those of X28 and X34, they are less prone to the formation of hot cracks
Table 2.
Chemical composition (%) of corrosion-resistant cast irons and cast steels
| Brand | WITH | Si | Μn | Cr |
| Chrome cast irons | ||||
| X28 X34 | 0,5-1,0 1,5-2,2 | 0,5—0,8 1,3-1,7 | 0,5—0,8 0,5—0,8 | 26—30 32—36 |
| Chrome steels | ||||
| 25X18L 30X20L | 0,20—0,30 0,25—0,35 | <0,8 <0,8 | <0,8 <0,8 | 17—20 20—23 |
| High silicon cast irons | ||||
| S15 S17 F15* | 0,5-0,8 0,3—0,5 0,5—0,0 | 14,5-16 16—18 15—16 | 0,3—0,8 0,3—0,8 0,3—0,8 | _ |
| Nickel cast iron** | ||||
| SCHShch-1 SCh1D-2 | 3,2—3,5 3,2—3,6 | 1,2-1,5 1,5—2,0 | 0,5—0,8 0,4—0,8 | 0,6—0,8 0,4—0,8 |
* Contains 3.5-4.5 Mo.
** Alloy SChShch-1 contains 0.8-1% Ni, alloy SChShch-2 0.4-0.5% Ni.
Chromium cast irons are resistant to oxidizing environments: in nitric acid of any concentration at 20°C and 40% boiling; in concentrated sulfuric acid and other media. Scale resistance is maintained up to 1000-1100° C.
Chromium cast iron is used to make parts and equipment for the nitrogen industry, artificial fertilizers, dies, etc. They are also used as heat-resistant materials - for the manufacture of furnace equipment, grates, combs and blades in furnaces intended for firing.
Silicon cast irons are acid-resistant alloys. Silicon, like chromium, expands the range of existence of ferrite and alloys containing up to 14.5% Si have the structure of a homogeneous solid solution. The carbon content in silicon cast iron is only 03-0.8%; with a higher content, carbon can be released in the form of graphite. Cast irons are smelted with a silicon content of up to 18%, since at a higher content these alloys become brittle and cannot be used. With a sharp change in temperature, cracking is possible. In oxidizing environments, a strong SiO2 film is formed on the surface of products, which is restored in case of mechanical damage.
Products from silicon cast iron are made only by casting, without subsequent mechanical processing (only grinding is possible).
The Φ15 alloy, also called "antichlor", contains 3.5-4.5% Mo. As a result of the addition of molybdenum, the alloy is stable in 10-30% solutions of hydrochloric acid (up to 90 ° C).
Silicon cast iron is used to make centrifugal pumps, acid sprayers, taps, boilers, vats, etc. All silicon cast iron has high oxidation resistance.
Nickel cast irons contain ~1% Ni (see Table 2). These cast irons are resistant to molten salts and concentrated alkali solutions. With increasing nickel content, the corrosion resistance of cast iron increases. The composition of nickel cast iron can be more complex: nickel-silicon austenitic cast iron contains,%: 1.7-2 C; 1.8-3 Cr; 5–7 Si and 16–20 Ni; nickel copper 2-2.8 C; 3-4 Cr; 5-8 Cu; 1.5-1 Si and 12-5 Ni.
Steel groups by chemical composition
Steels are classified according to the chemical elements that form them:
- Carbon steel grades.
- Alloyed.
Moreover, both of these groups are further divided according to the amount of carbon they contain into:
- Low carbon (carbon less than 0.3%).
- Medium carbon (carbon concentration is 0.3 - 0.7%).
- High carbon (carbon more than 0.7%).
A few words about steel quality
This parameter of a given alloy implies a set of properties, which, in turn, are determined directly by the process of its production. Similar characteristics that alloy tool steels are subject to include:
- Chemical composition.
- Uniformity of structure.
- Manufacturability.
- Mechanical properties.
The quality of any steel directly depends on how much oxygen, hydrogen, nitrogen, sulfur and phosphorus it contains. The method of producing steel also plays an important role. The most accurate method from the point of view of falling into the required range of impurities is the method of steel smelting in electric furnaces.
Industrial steels and cast irons
Industrial steel and cast iron are multicomponent alloys, which, in addition to iron and carbon, contain so-called banal impurities. Constant impurities are manganese, silicon, the presence of which is a technical characteristic of production, phosphorus, sulfur and oxygen-nitrogen, hydrogen, which cannot be completely removed from the metal. The content of carbon and impurities affects the properties of iron-carbon alloys.
Carbon has a great influence on the mechanical properties of steel.
The higher the carbon content of steel, the more cementite is contained in its structure.
Cementite is highly hard and brittle, so increasing the amount increases the strength and hardness of the steel, which reduces its ductility and strength. The carbon content of steel increases, density, electrical conductivity, thermal conductivity and permeability decrease, and electrical resistance increases.
Silicon and manganese are considered beneficial impurities. When making steel, it is added for deoxidation. When combined with iron oxide FeO, it turns into slag in the form of oxide. As a result of deoxidation, the properties of steel improve.
- If silicon remains in the steel after deoxidation, the yield strength will increase and the ability to work under cold pressure will decrease. Therefore, when stamping steel, it is necessary to reduce the silicon content.
- Manganese, without reducing ductility, significantly increases the strength of steel, sharply reduces its brittleness at high temperatures (red fracture), and removes sulfur from the melt.
Phosphorus and sulfur are harmful impurities. Phosphorus reduces the ductility and toughness of steel and facilitates its cracking at low temperatures (cold brittleness). Sulfur reduces the toughness, ductility, durability, weldability and corrosion resistance of steel. Sulfur causes embrittlement of steel at high temperatures. The content of sulfur and phosphorus in steel is strictly limited.
Alloy steel and changes in its properties
Alloy steel, the grades of which contain in their markings the letter designations of forcedly introduced elements, changes its properties not only from these third-party substances, but also from their mutual action with each other.
If we consider carbon specifically, then according to their interaction with it, alloying elements can be divided into two large groups:
- Elements that form a chemical compound (carbide) with carbon are molybdenum, chromium, vanadium, tungsten, manganese.
- Elements that do not create carbides are silicon, aluminum, nickel.
It is worth noting that steels that are alloyed with carbide-forming substances have very high hardness and increased wear resistance.
Low alloy steel (grades: 20KhGS2, 09G2, 12G2SMF, 12KhGN2MFBAYU and others). A special place is occupied by the 13X alloy, which is hard enough to make surgical, engraving, jewelry equipment, and razors from it.
Influence of alloying elements on properties
Tungsten increases the stability of austenite in the pearlitic region, but has little effect on the stability of austenite in the intermediate region. Increases resistance to tempering. It gives heat resistance. Manganese effect: Alloying ferrite involves strengthening it.
| Rapid cooling with water or an aqueous solution causes internal stresses that can cause cracks. |
| This is why it is recommended not to completely cool a carbon steel tool with water or an aqueous solution, but to cool it until the surface darkens (up to 200-250 ° C), and then transfer it to oil to cool completely. |
Manganese and chromium have the greatest impact on strength. In addition, the smaller the ferrite particles, the higher their strength. When the manganese content is more than 1%, hardness is improved, austenite zone is increased, hardenability is improved, deoxidation is enhanced, stabilized carbides are formed, and corrosion resistance is improved.
Abstracts on materials science
| The process of crystallization of metal melts. | Trends in the development of metallic materials. |
| Substitutional solid solutions. | Iron is an all-time favorite. |
Decoding
The content of alloying elements in steel can be determined by its marking. Each of these components introduced into the alloy has its own letter designation. For example:
- Chromium – Cr.
- Vanadium –V.
- Manganese –Mn.
- Niobium – Nb.
- Tungsten –W.
- Titanium – Ti.
Sometimes there are letters at the beginning of the steel grade index. Each of them carries a special meaning. In particular, the letter “P” means that the steel is high-speed, “W” indicates that the steel is ball-bearing, “A” is automatic, “E” is electrical, etc. High-quality steels have a number and letter designation at the end the letter “A”, and especially high-quality ones contain the letter “Ш” at the very end of the marking.
Impact of alloying elements
First of all, it should be said that carbon has a fundamental influence on the properties of steel. It is this element that, with increasing concentration, provides an increase in strength and hardness while reducing viscosity and plasticity. In addition, increased carbon concentration guarantees deterioration in machinability.
The chromium content of steel directly affects its corrosion resistance. This chemical element forms a thin protective oxide film on the surface of the alloy in an aggressive oxidizing environment. However, to achieve this effect, the steel must contain at least 11.7% chromium.
Aluminum deserves special attention. It is used in the process of alloying steel to remove oxygen and nitrogen after purging it in order to help reduce the aging of the alloy. In addition, aluminum significantly increases impact strength and fluidity, and neutralizes the extremely harmful effects of phosphorus.
Vanadium is a special alloying element that gives alloyed tool steels high hardness and strength. At the same time, the grain in the alloy decreases and the density increases.
Alloy steel, the grades of which contain tungsten, is endowed with high hardness and red resistance. Tungsten is also good because it completely eliminates brittleness during the planned tempering of the alloy.
To increase heat resistance, magnetic properties and resistance to significant impact loads, steel is alloyed with cobalt. But one of those elements that does not have any significant effect on steel is silicon. However, in those grades of steel that are intended for welded metal structures, the silicon concentration must necessarily be in the range of 0.12-0.25%.
Magnesium significantly increases the mechanical properties of steel. It is also used as a desulfurizer in the case of off-furnace desulfurization of cast iron.
Low-alloy steel (its grades contain alloying elements less than 2.5%) very often contains manganese, which provides it with an inevitable increase in hardness and wear resistance while maintaining optimal ductility. But the concentration of this element must be more than 1%, otherwise it will not be possible to achieve the specified properties.
Carbon steel grades, smelted for various large-scale building structures, contain copper, which provides maximum anti-corrosion properties.
To increase red-hardness, elasticity, tensile strength and corrosion resistance, molybdenum is necessarily introduced into the steel, which also increases the resistance to oxidation of the metal when heated to high temperatures. In turn, cerium and neodymium are used to reduce the porosity of the alloy.
When considering the influence of alloying elements on the properties of steel, one cannot ignore nickel. This metal allows steel to achieve excellent hardenability and strength, increase ductility and impact resistance, and lower the cold brittleness limit.
Niobium is also widely used as an alloying additive. Its concentration, 6-10 times higher than the amount of carbon necessarily present in the alloy, eliminates intergranular corrosion of stainless steel and protects welds from extremely unwanted destruction.
Titanium allows you to obtain the most optimal strength and ductility indicators, as well as improve corrosion resistance. Those steels that contain this additive are very well processed with various special-purpose tools on modern metal-cutting machines.
The introduction of zirconium into a steel alloy makes it possible to obtain the required grain size and, if necessary, influence grain growth.
Corrosion-resistant steel - main types
Corrosion-resistant alloys are defined by their ability to withstand exposure to a wide range of natural and artificial corrosive environments: atmospheric, underwater, soil (underground), alkaline, acidic, saline, and stray current environments. Resistance is manifested against the effects of chemical, electrochemical, and intercrystalline corrosion.
The classification of stainless alloys is regulated by GOST regulations, which describe the steel in accordance with production processes and applications.
Alloys are divided into several groups according to structure criteria. They differ in the percentage of carbon and the composition of alloying components. These relationships determine where and how a particular type of steel can be used.
Main groups:
- Ferritic
- Martensitic.
- Austenitic.
- Combined.
Ferritic group
The group of ferrites includes chromium steels. They are marked with the letter F. Steels with a high chromium content - up to 30%, and a low carbon content - up to 0.15%. They have ferromagnetic properties, that is, they are characterized by magnetization outside the magnetic field at a low critical temperature.
To achieve optimal properties, the balance between carbon and chromium content is adjusted and found.
Pros: high strength and equally high ductility.
Other characteristics:
- Good deformability under cold deformation conditions.
- High corrosion resistance.
- Can be heat treated by annealing.
Used in the production of rolled pipes, sheets and profiled intermediate and final products.
Industries using ferritic steels:
- Chemical and petrochemical industry. Equipment and structures for working in acidic and alkaline environments.
- Heavy engineering.
- Energy.
- Instrument making for industry.
- Production of household equipment and appliances.
- Food industry.
- Medical industry.
Examples of steel grades according to GOST and their applications:
Steel 08Х13 is a ferritic chromium alloy. Used for the production of cutlery.
Steel 12X13 is a ferritic chromium alloy. Used for storing alcohol-containing products.
Steel 12Х17 – ferritic chromium heat-resistant alloy. High-temperature processing of food products is carried out in containers made from it.
Martensitic group
Martensite refers to a structure that is obtained as a result of hardening a workpiece or ingot of metal and then tempering it. Hardening involves heating to a temperature that exceeds the critical temperature; tempering involves subsequent rapid cooling of the metal. As a result of this process, the crystal lattice is rearranged, making the material harder. But fragility may also increase.
This procedure produces alloys that combine
- High hardness.
- High strength.
- Good elasticity.
- Corrosion resistance.
- Heat resistance.
If you increase the carbon content of the alloy, the qualities of hardness and wear resistance increase.
The steel is intended for the manufacture of metal products for functioning in aggressive environments of medium and low intensity. The elasticity property makes it possible to manufacture equipment components such as springs, flanges, and shafts. Cutting elements - knives for structures in the chemical industry, as well as in the food industry - are made from martensitic and martensitic-ferritic combined steel.
Examples of steel grades according to GOST and their applications:
Steel 20Х13, 30Х13, 40Х13 – martensitic alloy. Used in the production of kitchen equipment.
Steel 14Х17Н2 is a martensitic-ferritic combined alloy, contains nickel. Used for the production of compressors and equipment for operation in aggressive environments and at low temperatures.
Austenitic group
The austenitic class of stainless steels is distinguished by its chemical structure, the introduction of carbon atoms into the molecular lattice of iron. Contains a large percentage of chromium and nickel – up to 33%. These are highly alloyed metals. Non-magnetic properties allow the alloys to be used in a wide range of production processes.
This determines such properties of the group of metals as
- Plasticity in cold and hot conditions.
- Strength.
- Weldability is excellent.
- Resistance to aggressive environments, an example of which is nitric acid.
- Ecological cleanliness.
- Resistance to electromagnetic radiation.
To obtain stable austenite, a face-centered crystal lattice of iron, steel is alloyed with nickel, increasing its content to 9%. Alloying is carried out with titanium and niobium to increase resistance to intergranular corrosion. Such alloys are called stabilized.
Corrosion-resistant steels of the group are difficult to machine metals. To make working with them easier, heat treatment methods are used: annealing and double hardening. Annealing is carried out by heating up to 1200 degrees. From about 3 o'clock. Cooling takes place in water or oil liquid, or in the open air. In this way, the flexibility of the alloy is increased by reducing hardness. Double hardening involves the process of normalizing a solid solution of metal at a temperature of 1200 degrees. C. Secondary hardening takes place at 1000 degrees. C. There is an increase in ductility and heat resistance - resistance to high temperatures.
Application
Austenitic metals are used for the production of structural materials for cold stamping and welding. They are made from:
- Various containers.
- Building construction.
- Pipes made of corrosion-resistant steel.
- Units for petrochemistry and chemical production.
- Structures for oil rigs, treatment stations.
- Mechanisms that operate underwater, such as turbines.
- Power devices in the energy sector.
- Components and assemblies for cars and aircraft.
- Equipment for food products.
- Medical, pharmacological equipment.
- Fastening elements.
- Welded structures.
- And other types of products.
Examples of steel grades according to GOST and their applications:
Steel 12Х18Н10Т is a high-alloy chromium alloy with nickel and titanium additives. It is used to make equipment for the oil refining and chemical industries.
Steel 12Х18Н10Т – austenitic chromium steel with nickel additive. Pipelines for the chemical and food industries with temperature restrictions are made from it.
Steel 12X15G9ND is a high-alloy alloy containing chromium, manganese, nickel, and copper. Used in the production of pipeline systems and containers working with organic acids of moderate aggressiveness
Combined alloys
They combine the structure and properties of the austenitic-martensitic or austenitic-ferritic categories.
Austenitic-ferritic steels contain a small amount of nickel, they have a high chromium content (more than 20%), alloying is carried out with niobium, titanium, and copper. After heat treatment, the ratio of ferrite and austenite becomes equilibrium. Such alloys are stronger than austenitic alloys, are distinguished by their ductility and resistance to intercrystalline corrosion. They withstand shock loads well.
Austenitic-martensitic group of metals with chromium content in the range of 12-18%, nickel in the range of 3.7-7.5%. Aluminum additives can be used. Hardening is carried out by hardening at a temperature of more than 975 degrees. C, and subsequent tempering at a temperature of 450-500 degrees. C. They have an increased yield strength: a characteristic that indicates the stress at which an increase in strain continues without an increase in load. The alloys demonstrate good weldability and good mechanical properties.