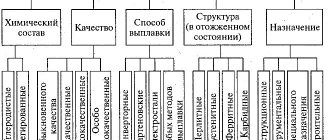
Composition of steel with carbon
Production technology does not completely remove impurities from steel. They occupy a small percentage, but are present in all carbon steels. Carbon content divides steel into carbon and alloy. Carbon is added intentionally to change the technical characteristics and mechanical properties of steels. The presence of impurities depends on the selected steel melt. The percentage of different elements in the steel composition:
- iron - up to 99%;
- carbon - up to 2.14%;
- silicon - up to 1%;
- manganese - up to 1%;
- phosphorus - up to 0.6%;
- sulfur - up to 0.5%.
Steel contains small amounts of hydrogen, oxygen and nitrogen.
Characteristic
The characteristics and structure of the metal are changed using heat treatment, through which the required surface hardness or other requirements for the use of the steel structure are achieved. However, not all structural properties can be adjusted using thermal methods. Such structurally insensitive characteristics include rigidity, expressed by the elastic modulus or shear modulus. This is taken into account when designing critical components and mechanisms in various fields of mechanical engineering.
In cases where the calculation of the strength of an assembly requires the use of small-sized parts that can withstand the required load, heat treatment is used. This effect on “raw” steel makes it possible to increase the rigidity of the material by 2-3 times. The metal that is subjected to this process is subject to requirements regarding the amount of carbon and other impurities. This steel is called high quality.
What are the properties of steel with different carbon contents?
The mechanical properties of steel depend on the amount of carbon. An increase or decrease in carbon content, even in hundredths of a percent, determines the scope of application of the metal. The structure of carbon steel varies depending on the content of cementite and ferrite. When more carbon is added to steel, the alloy becomes hard, strong and resilient. When reduced, its ductility and impact resistance are improved.
Depending on how much carbon is in the alloy, several types of steel are distinguished:
- Low carbon contains less than 0.25% carbon. Plastic, but easily deformable. Processed cold and at high temperatures.
- Medium carbon - 0.3-0.6%. Plastic, fluid and medium-strength. They are used to make parts and structures that will be used under normal conditions.
- High carbon - 0.6-2%. Wear-resistant, durable and expensive carbon steels with low toughness. They are difficult to weld without preheating the work area to +225°C.
Low-carbon and medium-carbon steels are easier to process and cook than high-carbon steels.
High carbon stainless steel
If you combine high carbon steel and stainless steel to make high quality carbon stainless steel, the composition of the metal will take the best of each alloy. This steel is resistant to rust or staining and is very hard. Generally, this alloy is considered to be a high quality stainless steel alloy.
Carbon stainless steel has a good edge when sharpened, and this hard metal is very suitable for knife making. A knife made of high-carbon stainless steel holds an edge well and for a long time, does not darken, and does not absorb foreign odors from prepared foods. It successfully combines the positive aspects of stainless and high-carbon steel and eliminates the disadvantages of each of them.
Types of carbon steel by degree of deoxidation
Carbon steel has different degrees of deoxidation. There are calm, boiling and semi-calm alloys. The names are associated with the content of harmful impurities - iron oxide. The less oxygen in the alloy, the more stable and durable the steel. After casting, the steel releases gases and hardens.
In calm steels, oxygen is almost completely removed, so they have a homogeneous structure and uniform distribution of composition. Semi-dormant ones often contain 0.15-0.3% carbon. Such steels are characterized by an uneven structure due to partial deoxidation of the alloy. Boiling steels have the most oxygen. This deoxidation results in a different chemical composition. Boiling steels contain many impurities: carbon, nitrogen, sulfur and phosphorus.
Use of high-quality structural carbon steel
The scope of application is quite wide. The main consumers of alloys are the engineering and construction industries. One of the advantages is considered to be good weldability.
As the name suggests, “structural” means used for building metal structures. Another name is reinforcing steel.
Considering the main grades of quality steel used by industrial enterprises, they can be divided by purpose.
- High-quality low-carbon steels 05-10. Their main purpose is the production of responsible and high-quality structures using welding (increasing the amount of carbon helps to reduce weldability). A small amount of carbon after welding does not provoke the formation of cracks in both hot and cold conditions.
- High-quality low-carbon steels 12-20. Their main purpose is the production of structural elements and parts that are not critical, lightly loaded, and subsequently cemented. Processed by cutting, cold stamping, complex drawing. Surface requirements: wear resistance, high hardness with a soft core. We manufacture mechanical engineering elements (shaft, axle, bolt, coupling, fork, lever, flanges and others), as well as elements of boiler equipment operating at high pressure and temperatures from -40°C to 450°C (pipeline, tee, connecting flange and others).
- High-quality medium carbon steels 25-35. Parts made from this material operate under medium loads and low stresses. After chemical-thermal exposure, they have high strength of the surface layer, wear resistance, but with insignificant strength of the core of the part (nut, screw, pawl, hook, cam, sprocket, etc.).
- High-quality medium carbon steels 40-45. After heat treatment, products made from this material can withstand medium loads (shaft, gear, connecting rod, etc.) well. To obtain blanks, the method of hot die stamping is used. Subject to all methods of heat treatment. In all medium-carbon steels, after quenching and subsequent high tempering, the internal structure becomes tempered sorbitol. In connection with this, viscosity increases with plasticity, and this means low sensitivity of stress concentrators. As the diameter of the product increases, its hardenability decreases.
- High-quality medium carbon steels 50-55. Parts made from these steels are highly loaded elements of mechanisms and assemblies (clutch, gear, spring ring, etc.).
- High-quality high-carbon steels 60-80 (G). Parts are manufactured that are subjected to constant compression stresses, which are operated under friction conditions (eccentric, spring, spring, etc.), as well as those operating under heavy dynamic and static loads (torsion bar, cross).
- High-quality boiler steel 12K-22K. They are used in the manufacture of parts whose operation involves elevated temperatures and high pressure. To improve weldability, titanium is introduced into the composition, and deoxidation is carried out by aluminum. Vessels and boilers that work with turbines, combustion chambers on ships and steam units are made from it.
- Automatic steel. Widely used in the industrial production of fasteners for automobiles and assemblies operating under static loads (bolt, nut, stud).
What is the difference between tool and structural steels?
The scope of application and manufacturing method are the main differences between steels. Structural carbon steels are smelted in converters and open-hearth furnaces. They come in high and ordinary quality. They are divided into groups A, B and C. They are marked with letters and numbers respectively. In the designation, the letter indicates the steel group, and the numbers indicate the carbon content, increased by 100 times. The higher the value, the stronger the steel. Ordinary quality steels with a high manganese content are marked with the letter “G”.
Steel of group A is supplied according to mechanical properties, group B - according to chemical composition, group B - according to mechanical properties and chemical composition. This means that group A steel has the declared properties, and group B steel meets the regulatory documentation.
Carbon tool steel is smelted in an open-hearth or electric furnace. It can be calm, semi-calm and boiling. It is divided into high-quality and high-quality steel. The proportion of impurities in high-quality tool steel is regulated: sulfur should be no more than 0.4%, phosphorus - no more than 0.6%. The number in the marking indicates the carbon content in hundredths. It also denotes the conditional number of the material brand.
The influence of carbon on the properties of steel
Carbon is not a random impurity, but the most important component of carbon steel, the amount of which determines its properties.
Machine-building plants receive steel from metallurgical plants in the annealed or hot-rolled state. The structure of structural steels (hypoeutectoid) consists of ferrite and pearlite, tool steels - of pearlite and cementite.
As the carbon content in the steel structure increases, the amount of cementite, a very hard and brittle phase, increases. The hardness of cementite exceeds the hardness of ferrite by approximately 10 times (800HB and 80HB, respectively). Therefore, the strength and hardness of steel increase with increasing carbon content, while ductility and toughness, on the contrary, decrease (Fig. 19).
As the carbon content increases to 0.8%, the proportion of perlite in the structure increases (from 0 to 100%), so both hardness and strength increase. But with a further increase in carbon content, secondary cementite appears along the boundaries of pearlite grains. In this case, the hardness almost does not increase, and the strength decreases due to the increased fragility of the cementite mesh.
In addition, an increase in carbon content leads to an increase in the cold brittleness threshold: every tenth of a percent increases t50 by approximately 20°. This means that steel with 0.4% C goes into a brittle state at approximately 0ºC, i.e. it is less reliable in operation.
The carbon content also affects all the technological properties of steel: the more carbon there is in the steel, the more difficult it is to process by cutting, the worse it deforms (especially in a cold state) and the worse it welds.
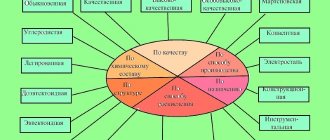
According to the quality category, carbon alloys of ordinary quality, high-quality, high-quality and especially high-quality are distinguished. The main signs of improved quality are more stringent requirements for the chemical composition and, above all, for the content of major harmful impurities, such as sulfur and phosphorus.
Quality is understood as a set of properties determined by the metallurgical production process. The uniformity of the chemical composition, structure and properties of steel, as well as its manufacturability, largely depend on the content of gases such as oxygen, nitrogen and hydrogen.
The designation of brands is alphanumeric.
Thus, carbon structural steels of ordinary quality (GOST 380-88) are marked with an alphanumeric code and, according to the guarantee of properties, upon delivery they are divided into three groups: A, B and C. The letters St mean steel, numbers from 0 to 6 are the conditional number of the brand, for example St0, St2, etc.
Group - A - alloys supplied with a guarantee of mechanical properties; their chemical composition is not regulated, it is only indicated in the certificates of the metallurgical manufacturing plant. They are used for the manufacture of machined parts.
Group B steels are supplied with a guarantee on the chemical composition, since they are usually subsequently subjected to various processing in order to obtain the set of mechanical properties required by the customer, namely hot pressure treatment and maintenance.
Alloys of group B are supplied with a guarantee for both the chemical composition and mechanical properties - according to the standards for steels of groups A and B. They are used in the production of welded structures.
The degree of deoxidation is denoted by the letters kp - boiling, ps - semi-calm and sp - calm. Steel grades St0 - St4 are boiling; all grades from St1 to St6 can be smelted as semi-calm and calm.
When marking, only groups B and C are indicated, for example St2kp or VStZps, which means steel 2, group A, boiling or steel 3, group B, semi-quiet, etc.
In high-quality alloys, the maximum content of harmful impurities is no more than 0.04% of sulfur and phosphorus. They are less contaminated with non-metallic inclusions and have a smaller amount of dissolved gases. They are supplied according to their chemical composition and mechanical properties.
Grades of high-quality carbon structural steels (GOST 1050-74 and GOST 4543-71) are designated by numbers indicating the average carbon content in hundredths of a percent, the degree of deoxidation by letters, for example, steel 10kp (this is 0.10% C, boiling); 20ps (0.20% C, semi-quiet). For mild steels there is no index.
Carbon high-quality tool alloys (GOST 1435-74) are marked with the letter - U, which means that the steel is carbon, and the following number indicating the average carbon content in tenths of a percent - 0.7 - 1.5%, for example U7, U7A, U13, U13A. High-quality alloys are characterized by the minimum possible amount of sulfur and phosphorus in them, less than 0.035%. To indicate high quality steel, a letter is placed at the end of the grade - A. For example, U7A, U13A, U10A.
Based on their structure in the annealed (equilibrium) state, the following groups of steels are distinguished:
1) technical iron with a carbon content of less than 0.02%. The alloy structure is single-phase – ferrite;
2) hypoeutectoid steels with carbon content from 0.02 to 0.8%. The structure of the alloys consists of ferrite and pearlite, and with increasing carbon content, the proportion of pearlite in the structure increases (Fig. 20.a);
3) eutectoid steel with a carbon content of 0.8%. The structure of the steel is pearlite: alternating plates of ferrite and cementite (Fig. 20, b, c);
4) hypereutectoid steels with carbon content from 0.8 to 2.14%. The structure consists of sections of perlite separated by brittle cementite shells (Fig. 20, d).
Fig. 20 Microstructures of steels:
a – hypoeutectoid steel (ferrite + pearlite); b – eutectoid steel (lamellar pearlite); c – eutectoid steel (granular pearlite); d – hypereutectoid steel (pearlite + secondary cementite).
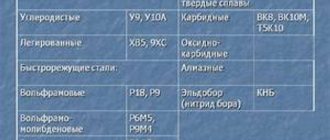
Alloy steels are iron-based alloys, into which chemical elements are specially introduced to provide it with the required structure and properties. In turn, alloy steels, depending on the number of alloying additives, are divided into single- and multi-component. More commonly used is the name indicating the alloying elements, for example, chromium steel, chromium-nickel steel, chromium-nickel-molybdenum steel, etc.
Usually the concentration of alloying additives is greater than the amount of these same elements in the form of impurities. According to the degree of alloying, i.e. according to the content of specially introduced additives, alloys are conventionally divided into low-, medium- and high-alloyed. The amount of these elements, in general, is 2.5 - 5.0%; up to 10% and more than 10%, respectively.
The concept of special steels is broader than alloyed alloys, since the former, in addition to alloyed ones, may also include carbon steels, which are given special properties through certain production and processing methods
Alloyed alloys (GOST 5632-72, GOST 20072-74) contain specially introduced alloying elements in various quantities, designated by the letters of the Russian alphabet: chromium - X, nickel - N, molybdenum - M, tungsten - B, cobalt - K, titanium - T, manganese - G, copper - D, vanadium - F, silicon - C, phosphorus - P, aluminum - U, cobalt-K, boron - P, niobium - B, zirconium - C, nitrogen - A. The numbers after the letter indicate the approximate content of this additive as a percentage, rounded to the nearest whole number. If there is no number after the letter, this means that the quantity of the element is less than or about 1.0%. The number 1 indicates that the concentration of the additive is from 1.5 to 2.0%.
The steel grade is indicated by a combination of letters and numbers. For structural grades, the first two digits indicate the average carbon content in hundredths of a percent. The number of alloying elements, if they exceed 1.0%, is placed after the corresponding letter in whole units. For example, 18KhGT steel contains about 0.18% carbon; 1.0% chromium, 1.0% manganese and about 0.1% titanium.
For steel alloyed with nitrogen, the letter A is placed in the middle of the grade designation, for example 15X17AGI4, but if it is placed at the end of the grade, this indicates that the alloy is high-quality - 30KhGSA. The letter - A, located at the beginning of the brand, indicates that the steel is automatic, with increased machinability, for example, A35G2.
Only alloyed iron-carbon alloys are of particularly high quality. They contain no more than 0.015% sulfur and 0.025% phosphorus. They are subject to high requirements regarding the content of other impurities.
Properties and composition of carbon steel, application and labeling
The scope of carbon steel is wide - it is used to create tools, load-bearing structures and elements for mechanical engineering are made from it. Currently, this is one of the most popular types of steel, as it has unique properties. Its operational and technical properties are determined by the components and their ratio in the composition.
Compound
Carbon and additional elements are used to melt steel.
Depending on the future purpose, certain requirements are imposed on the material: hardness, ductility, fluidity, etc. These parameters can be adjusted by changing the % carbon content. Its ratio to the total volume is one of the main conditions for dividing steel into types.
Their distinctive qualities and features are described in regulatory documents:
- Ordinary quality - GOST 380-85. Structural – GOST 380-88. Instrumental - GOST 1435-54 and GOST 5952-51.
The carbon content determines the hardness index. The more it is, the different the product will be. However, it must be taken into account that at the same time fragility increases.
Components of the structure of iron-carbon alloys
To analyze the effect of carbon content on iron-carbon alloys, it is necessary to characterize each of their structural elements. After slow cooling, steels consist of ferrite and cementite or ferrite and graphite.
Ferrite
Ferrite is ductile. In the annealed state, ferrite has a high elongation (about 40%). Its Brinell hardness ranges from 65 to 130 C depending on grain size and it is highly magnetic up to a temperature of 770 ˚C. At a temperature of 723 °C, ferrite dissolves 0.02% of carbon, and at room temperature, only thousandths of a percent of carbon remains in solution.
Cementite
Cementite is brittle and very hard. Its Brinell hardness is about 800. Cementite is a weak conductor of electric current and heat. It has a complex rhombic atomic lattice. Usually divided:
- primary cementite, which crystallizes from the liquid phase along the CD line;
- secondary cementite, which is released from the solid gamma solution along the ES line;
- tertiary cementite, which is released from the alpha solid solution along the PQ line.
Graphite
Graphite is soft. It is a poor conductor of electric current, but a good conductor of heat. Graphite does not melt even at a temperature of 3000-3500 ˚C. It has a hexagonal atomic lattice with an axial ratio greater than 2.
Austenite
Austenite is soft (but harder than ferrite) and ductile. The elongation of austenite is 40-50%. It has lower conductivity of heat and electricity than ferrite. Austenite is paramagnetic. It has a face-centric cubic lattice.