Hardening of hypoeutectoid and hypereutectoid steels
Until now, when talking about hardening, we were talking about eutectoid steel containing 0.8-0.9% C, and we have repeatedly noticed that carbon has a significant effect on the results. Let us now consider the effect of carbon on hardening in steels of hypoeutectoid and hypereutectoid composition.
First of all, in these steels, in comparison with eutectoid steel, their position in the state diagram should be affected: the presence of second transformations, in addition to the eutectoid one, at points A3 and Ast.
In this regard, in hypoeutectoid and hypereutectoid steels there can be two types of hardening: complete and incomplete.
Full hardening is called one that is carried out based on the state of continuous austenite, when the steel is heated for hardening above the upper critical points.
If we heat the steel below the indicated points, but above the Aclt point, then we will be in a region where, in addition to austenite, there are excess phases - ferrite (F) or cementite (C).
Obviously, during quenching, i.e., subsequent rapid cooling, the austenitic areas will supercool and transform into the corresponding hardened areas, and F and C will remain unchanged (equilibrium) areas. This type of hardening is called incomplete. The presence of equilibrium excess phases, along with hardened areas, should change the results of hardening. Therefore, to clarify the effect of carbon on hardening, we will first proceed from the complete hardening of steel.
Read also: Gold in radio components list
Comparing the results of complete hardening of steels with different carbon contents, it can be established that the process of decomposition of austenite and the formation of the corresponding transition states (martensite, troostite and sorbitol) proceeds similarly to eutectoid steel, but depending on the carbon content the following deviations are observed.
Regarding the isothermal decomposition curves, it has been established that changes in carbon content both in the hypoeutectoid side and in the hypereutectoid side shift the C-shaped curves to the left (closer to the initial ordinate), compared with the curves of eutectoid steel.
This means that eutectoid steel is the most stable, and a decrease and increase in carbon content compared to 0.83% causes an acceleration of both the beginning of the decomposition of supercooled austenite and the completion of the transformation.
As for the temperature limits for the location of the minimum stability (the inflection on the C-shaped curves in the upper part), no significant difference from eutectoid steel is observed here, and the course of the C-shaped curves is generally similar for all. The difference in the form of the isothermal transformation curves between eutectoid and extra-eutectoid steels is also reflected in the fact that, in addition to the pearlite transformation curve, they also contain a branch corresponding to the upper transformations: the release of excess ferrite (at points Arb) or cementite (Arcm).
The curves of the isothermal transformation of hypoeutectoid steel are shown and here a branch corresponding to the points of precipitation of excess ferrite Ar3 is visible; As cooling accelerates, these points become lower and closer to the Arx points - until they coincide with the latter near the C-shaped curve. This means that at high quenching rates (close to critical and higher), both transformations merge into one and give quenched structures without the presence of excess phases.
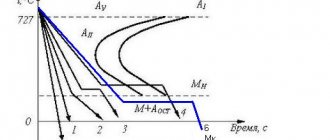
The difference due to carbon content is especially affected in the lower part of the diagram, on the position of the horizontal point M, which determines the onset of martensite formation (and limits the spread of C-shaped curves). It was noted above that both the upper and lower boundaries of the martensitic transformation region depend on the composition of the steel and, especially, on the carbon content. The curves showing the dependence of the start (M) and end (Mk) points of 1 martensitic transformation on the carbon content in steel; here it is clear that carbon sharply lowers these points, and
From these same curves it is clear that for low-carbon, very soft steels, the points of martensitic transformation are located so high that even if martensite is formed in them for a moment, it cannot remain so and must go into more stable stages of decomposition. Therefore, hardening of such steels for martensite is practically impossible.
In addition to carbon, other alloying impurities also have a significant influence on the position of martensite points, which is discussed in more detail.
Hypoeutectoid steel
When hardening, as a rule, one strives to obtain a martensite structure, which ensures maximum hardness of the steel. It is more expedient to obtain the sorbitol or troostite structure by tempering hardened steel. Hardening is the most complex type of heat treatment, since it occurs at very high cooling rates, which is associated with the formation of significant internal stresses in the metal.
Therefore, even small deviations from the established processing modes lead to defects in the product. The heating temperature and cooling rate of the product have a significant influence on the hardening results. Heating temperature of steel during hardening. When hardening steel, the heating mode is critical. To harden steel, it must be heated to a certain temperature. For example; If carbon steel is heated below the critical temperature Aci during hardening, then its hardness will change little compared to the initial state, and there will be no martensite in the structure of such steel. This is due to the fact that the steel was not heated enough. The martensite structure can only be obtained as a result of the transformation of austenite. In the steel discussed above, no austenite was obtained during heating, and therefore no martensite formation occurred during cooling.
Consequently, in order to harden steel, it must be heated above the critical temperature. The heating temperature of steel during hardening is determined primarily by its carbon content. When quenching, hypoeutectoid steels are heated to a temperature 40 - 50° above the critical temperature Asc. With such heating, the steel will receive an austenite structure, which, upon subsequent rapid cooling, will turn into martensite.
This hardening is called full hardening. During quenching, hypereutectoid steels are heated to a temperature 40–50° above the critical temperature Aci, i.e., up to 760–780°. At this temperature, the steel structure will consist of austenite and cementite. As a result of rapid cooling of the steel, austenite will transform into martensite, and cementite will remain in the structure of the hardened steel, since it does not undergo transformations during cooling.
Therefore, after quenching, the steel will have a martensite and cementite structure. This type of hardening is called incomplete. Why is incomplete quenching recommended for hypereutectoid steel and unacceptable for hypoeutectoid steel? If hypoeutectoid steel is subjected to incomplete quenching, that is, heated above the temperature Aci but below Acz, then in its structure there will be ferrite along with austenite. After hardening, the structure of such steel will consist of martensite and soft ferrite. The presence of ferrite in hardened steel reduces not only its hardness and strength, but also its plastic properties. Hypereutectoid steel after incomplete hardening has hard cementite in its structure, which not only does not reduce hardness, but, on the contrary, even improves the wear resistance of the steel. Complete hardening of hypereutectoid steel, i.e. heating it to a temperature above Ast, is dangerous and unnecessary.
At the same time, the hardness of the steel does not increase, but favorable conditions are created for overheating, the formation of hardening cracks and decarburization of the steel. Heating of products during hardening can be carried out in the same furnaces as during annealing. Small parts may become hot in salt baths.
The holding time of steel products at the quenching temperature must be sufficient to ensure the formation of homogeneous austenite over the entire cross-section of the product. The greater the thickness and weight of the product, the longer the exposure should be. However, excessively long exposure can lead to the growth of austenite grains. For products made of carbon steel, the holding time at the hardening temperature is set approximately within the following limits: for a thickness of 25 - 50 mm - 30 minutes, 50 - 75 mm - 45 minutes, 75-100 mm - 60 minutes.
- Surface hardening
Surface hardening is one of the ways to increase the hardness of the surface layers of a product. At the same time, abrasion resistance, endurance limit, etc. increase. Common to all methods of surface hardening is heating the surface layer of the part to the hardening temperature, followed by rapid cooling. These methods differ in the methods of heating products. The thickness of the hardened layer during surface hardening is determined by the depth of heating; hardenability plays a secondary role or does not matter at all.
Hardening with high frequency currents
(High-frequency hardening). The use of high-frequency waves for heating metals was first proposed by V. P. Vologdin in 1923. Hardening of steel with high-frequency heating began to be used in 1935. The theoretical foundations of heat treatment with high-frequency heating were developed in subsequent years by I. N. Kidin and N. V. Geveling , M. G. Lozinsky. The higher the current frequency, the thinner the hardened layer. Typically, in practice, machine generators with a frequency of 500-15,000 Hz and tube generators with a frequency of more than 106 Hz are used (the hardening depth at such frequencies is up to 2 mm). Inductors are made of copper tubes, inside which water continuously circulates, so they themselves do not heat up. The shape of the inductors corresponds to the external shape of the product, and it is necessary to maintain a constant distance between the inductor and the surface of the product. Each installation has a set of inductors. The HDTV part heats up in 3-5 s. After heating in the inductor, the part is quickly moved into a special cooling device - a spray, through the holes of which the quenching liquid is sprayed onto the heated surface (sometimes the heated parts are dumped into quenching tanks). A high heating rate shifts phase transformations to higher temperatures. In addition, due to short exposures, the diffusion of carbon does not have time to occur and in the resulting austenite, inhomogeneity of its distribution is observed. To speed up the diffusion processes, the heating temperature is increased. Therefore, the quenching temperature during HFC heating for the same steel should be higher than during conventional heating. Under the correct conditions, finely needle-shaped or structureless martensite is obtained, which has less brittleness and increased strength. Hardness increases by 2-3 units compared to conventional hardening, and wear resistance and endurance limit also increase, which can increase by 1.5-2 times. Since the core of the product is heated below Ac1 when heated by high-frequency frequencies, it is subjected to normalization before hardening to improve its properties. It is most advisable to use this method for heating products made of carbon steels containing more than 0.40% C. For alloy steels, high-frequency heating, as a rule, is rarely used, since one of their advantages - the deep hardenability of alloy steels - is not used with this method.
Advantages of the HDTV method
— high productivity, absence of decarburization and oxidation of the surface of the part, the ability to regulate and control the heat treatment regime, as well as complete automation of the entire process. Hardening units can be installed directly in the production line of a machine shop. Therefore, high-frequency hardening is used for mass-produced parts (fingers, rollers, gears, etc.). To avoid possible brittle fracture of gear teeth, they are made from special carbon steels of reduced hardenability 55PP (0.55% C), containing less manganese (<=0.2%) and silicon (0.1 - 0.3%). When heated, the gear teeth heat up through and through, but only the surface layer 1-2 mm thick is hardened. High-frequency heating allows for hardening of individual sections of parts - crankshaft journals, camshaft cams, rail heads, etc. The disadvantage is the high cost of induction installations and inductors (each part has its own inductor), so this method is economically feasible to use only in mass production similar parts of simple shape.
Flame surface hardening
used mainly for hardening products with a large surface, in individual production and repair, sometimes for hardening steel and cast iron rolling rolls. The products are heated by the flame of gas or oxygen-acetylene burners. When heating products with a large surface, the burners with a cooling device move along the product or the product moves, but the heating device is stationary. The thickness of the hardened layer with this heating method is 2-4 mm. The disadvantages of the method include the difficulty of regulating the heating temperature, and hence the possibility of severe overheating.
Heating products before quenching in molten metals or salts also
is one of the methods of surface hardening. This method is used when hardening small parts of simple geometric shapes produced in small quantities.
Hardening defects. Warping, hardening cracks, change in product shape
— these defects are a consequence of the occurrence of internal stresses of the first kind. One way to reduce the formation of these defects is to slowly cool the parts in the martensitic transformation temperature range.
Incomplete hardening
— after hardening, insufficient hardness is obtained. This defect is formed either as a result of underheating before hardening (for example, when heating hypoeutectoid steel below Ac3), or as a result of cooling at a rate less than critical (see Fig. 124, speed v7). This defect can be eliminated by repeated hardening under the correct conditions.
Overheat
- hardening at high temperatures.
The result is coarse-needle martensite, and the products have increased fragility. Soft spots on the surface of the part
(i.e., areas with reduced hardness) are the result of the formation of a steam jacket on the surface of the part during hardening, which reduces the cooling rate. The defect is corrected by repeated hardening.
Oxidation and decarbonization of the product surface
- this defect occurs as a result of the interaction of the furnace atmosphere with the surface layers of the part during heating. Eliminated as a result of carrying out the correct heat treatment regime or heating carried out in neutral atmospheres (nitrogen, argon, etc.).
- Types of permanent connections
Permanent connections are those that can be made only by destruction or unacceptable residual deformation of one of the structural elements. These include welded, riveted, soldered, and adhesive joints.
1. Welded joints
A welded joint is a set of products connected by welding.
Welding is the process of obtaining a permanent connection of parts by directing metal to form a weld at the joints. A weld is a seam that has solidified after the material has melted. The most widespread are gas, arc and resistance welding.
Designation of standardized welding methods
GOST
| Method name | Legend | |
| 5264–80* | Manual arc welding | R |
| 8713–79* | Automatic welding under a layer of flux without the use of backing plates, pads and weld seams The same, using a flux pad The same, using a steel lining Semi-automatic welding under a layer of flux without the use of linings, pads and manual welding The same, using a steel lining | A Af Ace P Ps |
| 11533–75* | Automatic submerged arc welding (acute and obtuse angles) with manual welding Semi-automatic submerged arc welding (acute and obtuse angles) with manual welding | Ar Pr |
| 15878–79 | Contact welding: Spot Roller Embossed Butt | CT Kr Kv KS |
| 15164–78* | Electroslag welding with wire electrode | Shae |
| 14771–76* | Electric arc welding of shielding gases in inert gases with a non-consumable electrode, in carbon dioxide with a consumable electrode | IN UP |
| 14806–80* | Electric arc welding of aluminum in aluminum alloys in inert gases | AINp |
| 16310–80* | Welded connection made of polyethylene, polypropylene and vinyl plastic | G, E |
There are front and back sides. In a one-sided seam, take the side from which welding is performed as the front side; in a double-sided seam with an asymmetrical bevel - the side from which the main seam is welded; in a double-sided seam with a symmetrical bevel - either side.
Depending on the relative position of the welded parts, the following types of welded joints are distinguished:
butt (C) - parts to be welded are connected along their end surfaces; angular (U) – the parts to be welded are located at an angle and are connected along the edges; T-bar (T) - the end of one part is connected to the side surface of another; overlap (N) – the side surfaces of the parts being connected partially overlap each other.
According to their length, welds can be:
- continuous along a closed loop;
- along an open contour;
- intermittent.
Intermittent seams have welded sections of equal length with equal intervals between them.
In double-sided welding, if the welded sections are located opposite each other, such a seam is called a chain seam, if the sections alternate, then the seam is called a checkerboard seam.
The designation structure for standard seams is as follows:
- auxiliary signs (O – seam along a closed line, ? – assembly seam);
- standard number;
- standard alphanumeric designation of a seam;
- standard symbol for welding method;
- auxiliary sign ? – triangle size of the seam leg;
- seam size in mm;
- auxiliary signs (tab);
- designation of weld surface roughness;
- instructions for seam inspection.
The seams of welded joints can be made reinforced.
The reinforcement (convexity) of the seam is determined by the value q.
Some types of seams are characterized by a K value, called the seam leg. The ? sign is placed in front of the leg size.
Examples of weld symbols