The influence of the main alloying elements on the properties of steel.
The influence of individual components on the properties of steel
Alloy steel is a steel that, in addition to the usual impurities, contains alloying elements specially introduced in certain combinations (Cr, Ni, Mo, Wo, V, A1, B, Ti, etc.), as well as Mn and Si in quantities exceeding their usual content as process impurities (1% and above). As a rule, complex alloying provides the best properties.
Alloying of steels and alloys is used to improve their technological properties. Alloying can increase the yield strength, impact strength, relative contraction and hardenability, and also significantly reduce the hardening rate, the cold brittleness threshold, the deformability of products and the possibility of crack formation. In products with large sections (diameter over 15–20 mm), the mechanical properties of alloy steels are significantly higher than the mechanical properties of carbon steels.
Impact of impurities
Permanent (technological) impurities are essential components of steels and alloys, which is explained by the difficulty of removing them as during smelting (P, S). So in the process of deoxidation (Si, Mn) or from a charge - alloyed scrap metal (Ni, Cr, etc.).
Permanent impurities include carbon, manganese, silicon, sulfur, phosphorus, as well as oxygen, hydrogen and nitrogen.
Carbon
With an increase in carbon content to 1.2%, strength, hardness, cold-brittleness threshold increase (0.1% C increases the temperature of the cold-brittleness threshold by 20C), yield strength, electrical resistance and coercive force. At the same time, density, thermal conductivity, viscosity, plasticity, values of relative elongation and contraction, as well as the value of residual induction are reduced.
A significant role is played by the fact that a change in physical properties leads to a deterioration in a number of technological characteristics - such as deformability during stamping, weldability, etc. Thus, low-carbon steels are characterized by good weldability. Welding medium and especially high-carbon steels requires the use of heating, which slows down cooling, and other technological operations that prevent the formation of cracks.
Manganese
Manganese is introduced into steel as a technological additive to increase the degree of their deoxidation and eliminate the harmful effects of sulfur. Manganese is considered a technological impurity if its content does not exceed 0.8%. Manganese as a technological impurity does not have a significant effect on the properties of steel.
Silicon
Silicon is also introduced into steel for deoxidation. The silicon content as a technological impurity usually does not exceed 0.37%. Silicon as a technological impurity does not affect the properties of steel. In steels intended for welded structures, the silicon content should not exceed 0.12-0.25%.
Sulfur
The limits for sulfur content as a technological impurity are 0.035-0.06%. An increase in sulfur content significantly reduces the mechanical and physicochemical properties of steels, in particular, ductility, impact strength, abrasion resistance and corrosion resistance. During hot deformation of steels and alloys, a high sulfur content leads to red brittleness. In addition, increased sulfur content reduces the weldability of finished products.
Phosphorus
The limits for phosphorus content as a technological impurity are 0.025-0.045%. Phosphorus, like sulfur, is one of the most harmful impurities in steels and alloys. An increase in its content, even by a fraction of a percent, while increasing strength, simultaneously increases fluidity, fragility and the threshold of cold brittleness and reduces ductility and viscosity. The harmful effects of phosphorus are especially pronounced at high carbon levels.
Oxygen and nitrogen
Oxygen and nitrogen dissolve in negligible quantities and contaminate steel with non-metallic inclusions (oxides, nitrides, gas phase). They have a negative impact on properties, causing an increase in brittleness and cold brittleness threshold, and also reduce viscosity and endurance. When the oxygen content is more than 0.03%, steel aging occurs, and more than 0.1% - red brittleness. Nitrogen increases the strength and hardness of steel, but reduces ductility. An increased amount of nitrogen causes strain aging. Aging develops slowly at room temperature and accelerates when heated to 250oC.
Hydrogen
An increase in its content in steels and alloys leads to an increase in brittleness. In addition, flakes may appear in rolled products, which are developed by hydrogen released into the pores. Flocks initiate the destruction process. Metal with flakes cannot be used in industry.
Influence of alloying elements
Alloying of steels and alloys is used to improve their technological properties. Alloying can increase the yield strength, impact strength, relative contraction and hardenability, and also significantly reduce the hardening rate, the cold brittleness threshold, the deformability of products and the possibility of crack formation. In products with large sections (diameter over 15-20 mm), the mechanical properties of alloy steels are significantly higher than the mechanical properties of carbon steels.
All alloying elements, with the exception of nickel, when contained in a solution above a certain limit, reduce impact strength, crack resistance and increase the cold brittleness threshold.
Classification
Based on their applicability for alloying, three groups of elements can be distinguished. The applicability of various elements for alloying is determined not so much by physical, but mainly by economic considerations.
Alloying elements according to the mechanism of their effect on the properties of steels and alloys can be divided into three groups:
- influence on polymorphic (alpha-Fe -> gamma-Fe) transformations;
- formation of carbides (Cr,Fe)7C3 with carbon; (Cr,Fe)23C6; Mo2C and others;
- formation of intermetallic compounds (intermetallic compounds) with iron – Fe7Mo6; Fe3Nb, etc.
Based on the nature of their influence on polymorphic transformations, alloying elements can be divided into two groups:
- elements (Cr, W, Mo, V, Si, Al, etc.), a sufficient content of which ensures the existence of alloyed ferrite (ferrite compounds) in steels at all temperatures;
- elements (Ni, Mn, etc.) that stabilize alloyed austenite at a sufficient concentration at all temperatures (austenitic alloys). Alloys that only partially undergo the gamma->alpha transformation are called semi-austenitic or semi-ferritic, respectively.
Alloying ferrite is accompanied by its hardening. Manganese and chromium have the most significant effect on its strength. Moreover, the finer the ferrite grain, the higher its strength. Many alloying elements help to refine the ferrite and pearlite grains in steel, which significantly increases the toughness of the steel. However, all alloying elements, with the exception of nickel, when contained in a solution above a certain limit, reduce impact strength, crack resistance and increase the cold brittleness threshold. Nickel lowers the threshold of cold brittleness. Alloyed austenite is paramagnetic and has a high coefficient of thermal expansion. Alloying elements, including nitrogen and carbon, the solubility of which in austenite at normal temperatures reaches 1%, increase its strength at normal and high temperatures and reduce the yield strength. Alloyed austenite is the main constituent of many corrosion-resistant, heat-resistant and non-magnetic alloys. It is easily hardened, that is, it is quickly and strongly strengthened under the influence of cold deformation. Alloying elements (with the exception of cobalt), increasing the stability of austenite, reduce the critical hardening rate and increase hardenability. For many austenitic alloys, the critical hardening rate is reduced to 20°C/s and below, which is of great practical importance. Carbide-forming elements: Fe – Mn – Cr – Mo – W – Nb – V – Zr – Ti (with the exception of manganese) prevent the growth of austenite grains when heated. Steel alloyed with these elements, at the same temperature, retains a higher dispersion of carbide particles and, accordingly, greater strength. Intermetallic compounds are formed when there is a high content of alloying elements between these elements or with iron. Examples of such compounds are Fe7Mo6, Fe3Nb2, etc. Intermetallic compounds, as a rule, are distinguished by increased hardness and brittleness.
The influence of chromium on the properties of steels
The tendency of chromium to form carbides is average among other carbide-forming alloying elements. At a low Cr/C ratio of chromium content relative to iron, only cementite of the type (Fe,Cr)3C is formed. With an increase in the ratio of chromium to carbon content in Cr/C steel, chromium carbides of the form (Cr,Fe)7C3 or (Cr,Fe)23C6 or both appear. Chromium increases the ability of steels to be thermally hardened, their resistance to corrosion and oxidation, provides increased strength at elevated temperatures, and also increases the abrasive wear resistance of high-carbon steels.
Chromium carbides are also wear-resistant. They are the ones who provide durability to steel blades - it’s not for nothing that knife blades are made from chrome steels. Complex chromium-iron carbides enter the solid solution of austenite very slowly - therefore, when heating such steels for hardening, a longer exposure at the heating temperature is required. Chromium is rightfully considered the most important alloying element in steels. The addition of chromium to steels causes impurities such as phosphorus, tin, antimony and arsenic to segregate to the grain boundaries, which can cause temper brittleness in steels.
Basic properties and importance of silicon
Silicon
Silicon has the following physical and chemical properties: atomic weight 28.06; density 2.4 g/cm 3 ; melting point 1414°C; boiling point 2287°C; heat of fusion 39.76 kJ/mol. In liquid iron, silicon has unlimited solubility, in solid iron it has limited solubility (up to 14%). Silicon forms several compounds with iron - Fe3Si2, FeSi and FeSi5, but only silicide
FeSi (33.3% Si), having a melting point of 1410°C. An oxygen compound of silicon that is stable in steel-smelting baths is SiO2 (melting point 1710°C).
Silicon is one of the most common in nature and ranks second after oxygen (26% Si in the earth's crust). Due to its high chemical affinity for oxygen and its high availability, silicon is primarily used as a deoxidizer in steel production. In addition, silicon is introduced into the metal to alloy it.
For deoxidation, silicon is usually added to mild steel in an amount of 0.15–0.35%, and to semi-mild steel – up to 0.10–0.12%. In boiling steel, silicon is an undesirable impurity that worsens the boiling of the metal in the mold and the structure of the ingot, therefore the silicon content in boiling steel should not exceed 0.02-0.03%.
Silicon as an alloying element in steels is contained in an amount of 0.5-0.6% or more.
Steel alloyed with silicon has higher values of yield strength, elasticity, impact resistance, low residual magnetism, good hardenability, heat resistance, the ability to maintain hardness in a hardened state at relatively high temperatures and other useful properties. Steels for various purposes are alloyed with silicon: structural (0.8–1.5% Si), tool (1.2–1.6% Si); spring-spring (1.3-2.0% Si), heat- and scale-resistant (2.0-3.0% Si), dynamo-transformer (2.5-4.5% Si), etc. Usually steel doped with silicon in combination with other impurities, most often in combination with chromium and manganese.
The silicon contained in the metal charge, although oxidized and lost almost completely during smelting, usually has a positive effect on the course of the process. This is reflected in an improvement in the heat balance of the melt, since among the usual impurities of the metal charge, silicon is oxidized with the release of the greatest amount of heat.
In any steelmaking slag, silica is one of the most important components. Silica, resulting from the oxidation of silicon in a bath, is more active when added in finished form and accelerates the process of slag formation. However, silica, formed during the oxidation of metal silicon, has a destructive effect on the main lining, especially in processes with high consumption of liquid iron, for example in converter processes. In addition, very high silicon contents produce large amounts of slag, which is not always desirable, so limits are usually set for the silicon content of cast iron. For example, for the main open-hearth and oxygen-converter processes, it is desirable to have the silicon content in cast iron in the range of 0.5-0.8%.
Behavior of silicon in steel melting baths. Silicon is an essential impurity of cast iron and is contained in scrap in varying quantities. Typically, the silicon content in the metal charge is quite high (0.5-1.0%). Silica is a strong acidic oxide, therefore the completeness of the silicon oxidation reaction also depends on the type of process, more precisely, the nature of the slag under which the smelting is carried out.
In the main processes , silica forms strong compounds in the slag: at the beginning of smelting, iron silicates 2FeO•SiO2 and calcium CaO•SiO2, later calcium silicate 2CaO•SiO2. Due to this, the activity of SiO2 in the slag is very low even at its high concentration, and silicon in the main processes is oxidized almost completely at the beginning of melting, and during melting it is not restored in noticeable quantities, regardless of the presence of carbon and other common impurities of cast iron and changes in bath temperature. At the beginning of smelting, silicon oxidation is promoted by the relatively low bath temperature and high FeO content in the slag. As melting progresses, the temperature of the bath rises. This causes the reaction to shift to the left, towards the reduction of silicon, since the reaction is exothermic. However, as the temperature of the bath increases, the basicity of the slag simultaneously increases, which contributes to a deeper desiliconization of the metal with the formation of the most durable calcium silicate 2CaO•SiO2. As a result of the action of these two opposing factors, the residual silicon content in the metal remains approximately at the same level, usually 0.01-0.02%.
Scope of metal application
The main area where it is used is metallurgy, where it is used as additional components in the manufacture of high-alloy stainless steels. When nickel is added to the molten iron, very high-quality, quite strong, but at the same time flexible alloys with increased resistance to corrosion are subsequently obtained.
Nickel alloy is widely used in the following areas:
- industrial sector
- food and chemical fields
- petrochemical direction
- furniture area
- medical field (pharmaceuticals)
- aviation and mechanical engineering
- in the production of cables used in the production of heating parts used in industrial devices
- for machines and specialized equipment
- in the process of producing interior parts
- at the time of production of household utensils, including household appliances
Nickel alloys have the unique ability to retain their qualities even after repeated and prolonged heating.
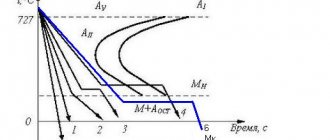
The influence of chem. elements on the properties of steel.
Symbols of chemical elements:
| chromium (Cr) - X nickel (Ni) - H molybdenum (Mo) - M titanium (Ti) - T copper (Cu) - D vanadium (V) - F tungsten (W) - B | nitrogen (N) - A aluminum (Al) - Yu beryllium (Be) - L boron (B) - P bismuth (Bi) - Vi gallium (Ga) - Gl | iridium (Ir) - And cadmium (Cd) - Kd cobalt (Co) - K silicon (Si) - C magnesium (Mg) - Ш manganese (Mn) - G | lead (Pb) - AC niobium (Nb) - B selenium (Se) - E carbon (C) - U phosphorus (P) - P zirconium (Zr) - C |
INFLUENCE OF IMPURITIES ON STEEL AND ITS PROPERTIES
Carbon - found in steel usually in the form of a chemical compound Fe3C called cementite. With an increase in carbon content to 1.2%, the hardness, strength and elasticity of steel increase, but ductility and impact resistance decrease, and workability deteriorates, and weldability also deteriorates.
Silicon - if it is contained in steel in small quantities, it does not have a special effect on its properties. (Useful impurity; introduced as an active deoxidizer and remains in the steel in an amount of 0.4%)
Manganese , like silicon, is found in ordinary carbon steel in small quantities and does not have any particular effect on its properties. (A useful impurity; introduced into steel for deoxidation and remains in it in an amount of 0.3-0.8%. Manganese reduces the harmful effects of oxygen and sulfur.
Sulfur is a harmful impurity. It is found in steel mainly in the form of FeS. This compound makes the steel brittle at high temperatures, such as during forging, a property called red brittleness. Sulfur increases the abrasion of steel, reduces fatigue resistance and reduces corrosion resistance. In carbon steel, no more than 0.06-0.07% sulfur is allowed. (Steel is protected from red brittleness by manganese, which binds sulfur into MnS sulfides).
The influence of silicon on the structure and properties of high-manganese steels
50>
Silicon is introduced into 110G13L steel, as well as into carbon steels, in the amount necessary for deoxidation. The positive role of silicon also lies in improving the casting properties of steel: an increase in its content from 0.17 to 1.11% leads to an improvement in fluidity by approximately 1.5 times, which reduces defective defects due to lack of welding and underfilling and has a positive effect on the quality of castings of complex shapes. In addition, increasing the silicon concentration in high-manganese steel from 0.2 to 1.2% reduces its shrinkage from 2.9 to 2.3% and improves the crack resistance of castings ,
which is associated with the formation of silicides of iron, manganese and silicon nitrides in a solid solution of silicon substitution in manganese austenite, which inhibit the shrinkage of steel.
Regarding the influence of silicon on the level of mechanical and other characteristics of steel, the opinions of various authors differ. It is obvious that these discrepancies are caused by the multifactorial influence of silicon on the structure of steel at the micro, meso and macro levels. It is known that the degree of strengthening of a solid solution when substituting atoms are introduced into it depends on a number of factors, the most important of which is the mismatch parameter - the difference in the sizes of solvent atoms and dissolved atoms and a change in the electronic structure of the solid solution. When silicon is dissolved in ferrite, the mismatch parameter due to the small atomic radius of silicon is maximum among the substitution alloying elements, which also causes the maximum efficiency of this element as a hardener of α-iron. In austenitic steels, silicon also reduces the lattice parameter of the solid solution, which causes its hardening. For this reason, increasing the silicon concentration to a certain value (approximately 1.2%), the strength characteristics of 110G13L steel increase with practically unchanged ductility characteristics, and when these limits are exceeded, all properties decrease. A number of researchers believe that when the silicon content in steel is up to 0.8–1.0%, it has little effect on the level of mechanical and other characteristics, since large carbides are rarely found in the metal structure with such a silicon content, and starting from this concentration , reduces the strength and plastic properties, impact strength and cold resistance of steel.
For example, according to Uralmashplant, with a silicon content in steel of 0.6–0.9%, the impact strength was 1.5–2.2 MJ/m2, and with a concentration of 1.4–2.3%, it was only 0.3– 1.0 MJ/m2.
Another group of authors is of the opinion that an increase in silicon concentration to 1% (within GOST 977-88, 21357-87) reduces the strength characteristics, impact strength and wear resistance of steel, and increases defective hot cracks. Thus, when the silicon content in 110G13L steel increases from 0.17 to 1.11%, its impact strength decreases by 4 times, and the relative elongation and contraction decreases by 1.5 times. The harmful effect of silicon is especially pronounced at high (above 1.2%) carbon content. According to N. G. Davydov, steel containing 0.35–0.60% Si has the highest impact strength and best cold resistance, and a further increase in the silicon concentration reduces the impact strength both at room and at negative temperatures (Fig. 4.49) .
Rice. 4.49. Effect of silicon content
for impact strength of steel 110G13L
According to most authors, silicon reduces the solubility and reduces the diffusion coefficient of carbon in manganese austenite. The result of this is an increase in the number and thickness of carbides during the crystallization of steel with a high silicon content and their concentration due to carbon segregation, mainly in the boundary regions of grains and directly at the boundaries. According to O.D. Moldavsky and others, as the silicon content increases from 0.20% to 0.76%, carbides turn from acicular to spheroidal. Silicon promotes the preservation of carbides in steel when it is heated for hardening and the release of carbides when heating hardened steel.
To dissolve a large number of large carbides in austenite during heating for quenching, a higher temperature and longer exposure are required, which contributes to decarburization of the surface of the castings and oxidation of the metal along the grain boundaries. The dissolution of large boundary carbides causes the appearance of micropores, which subsequently serve as sources of microcracks.
The segregation of phosphorus in the presence of silicon also increases and thereby increases its release at grain boundaries in the form of phosphide eutectic, which reduces the mechanical and operational properties of steel. In addition, with increasing silicon concentration, the grain size of 110G13L steel increases. This is especially pronounced at high metal pouring temperatures, and in steels with low silicon (≤ 0.6%) a satisfactory grain size can be obtained even at elevated pouring temperatures. The increased silicon content in high-manganese steel promotes the formation of a dendritic and coarse-grained structure in castings and can lead to a sharp decrease in ductility, which makes this steel unsuitable for use under dynamic loading conditions, especially at subzero temperatures.
50>
Date added: 2020-04-13; views: 401; ORDER A WORK WRITING