Electrode wire: brands, designation, supply
The chemical composition of the electrode wire determines the composition of the weld metal and, consequently, its mechanical properties.
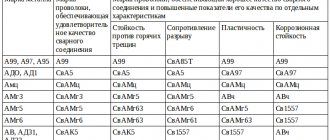
Steel welding wire manufactured in accordance with GOST 2246-70, which provides for 77 grades of wire.
The symbols for wire grades include the index St (welding) and the numbers and letters that follow it. The numbers after the C index indicate the average carbon content in hundredths of a percent.
Just as in steel grades, alloying elements in wire grades are designated by letters:
- A - nitrogen;
- Yu - aluminum;
- P - boron;
- F - vanadium;
- B - tungsten;
- K - cobalt;
- C—silicon;
- G - manganese;
- D - copper;
- M - molybdenum;
- N - nickel;
- B - niobium;
- E - selenium;
- T - titanium;
- X - chrome.
The numbers following the letter designations of chemical elements indicate the average content of the element as a percentage. If the content of the alloying element is less than 1%, then only the corresponding letter is indicated.
The letter A at the end of the symbols for grades of low-carbon and alloyed wires indicates increased purity of the metal in terms of sulfur and phosphorus content. SV-08AA wire contains no more than 0.020% sulfur and no more than 0.020% phosphorus.
In the symbol of welding wire, before the index Sv, a number indicating the diameter of the wire in mm is indicated, and after the symbol, the GOST number is indicated.
For example: a welding wire with a diameter of 3 mm, brand Sv-08A, intended for welding (surfacing), with a non-copper-plated surface is conventionally designated as follows: wire 3 Sv-08A GOST 2246-70.
If the wire is supplied with a copper-plated surface, then the letter O is placed after the wire grade.
The letter E means that the wire is intended for the manufacture of electrodes.
The letters Ш, ВД or VI indicate that the wire is made of steel melted by electroslag or vacuum arc remelting, or remelting in vacuum induction furnaces.
Welding wires are divided into:
- low-carbon (with a total content of alloying elements up to 2%);
- alloyed (total content of alloying elements from 2 to 6%) and highly alloyed (total content of elements more than 6%).
The wire is supplied in coils weighing up to 80 kg. A metal tag indicating the manufacturer, wire symbol, batch number and technical control mark is attached to each coil. By agreement of the parties, the wire can be supplied wound on reels or cassettes.
The wire should be transported and stored under conditions that prevent rusting, contamination and mechanical damage. If the surface of the wire is dirty or covered with rust, it must be cleaned before use. The wire is cleaned when winding it onto cassettes in special machines using sanding wheels. To remove oils, kerosene, white spirit, gasoline, etc. are used. To remove moisture, heat treatment is used: calcination at a temperature of 100 - 150 ° C. It is also recommended to treat the wire in a 20% solution of sulfuric acid, followed by calcination at a temperature of 250°C for 2-2.5 hours. There is no need to treat the electrode wire before welding if you use copper-plated wire.
In accordance with the requirements of EN 756, the designation of welding wires is based on the following scheme:
| S.A. | X; X/2 | H(L) | Si(Si2) | Mo(Mo1) | Ni (Ni0.5; Ni1; Ni 2) |
| Solid wire for submerged arc welding | [Mn],% | H? C > 0.1 L ? C 0.1 | Si? Si = 0.3 Si2 ? Si = 0.6 | Mo? Mo Mo1 ? Mo = | Ni? Ni 0.5; Ni0.5 ? Ni = 0.4…0.8; Ni1? Ni = 1.0…1.5 |
Welding fluxes: functions, classification, general requirements
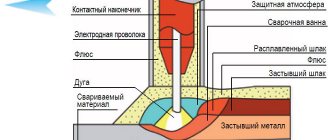
Welding flux is one of the most important elements that determine the quality of the weld metal and the conditions for the welding process. The composition of the liquid slag and gas atmosphere depends on the composition of the flux. The interaction of slag with metal determines the specific chemical composition of the weld metal. The composition of the weld metal determines its structure and resistance to cracking. The composition of the gas atmosphere determines the stability of the arc, resistance to the appearance of pores and the amount of harmful gases released during welding.
Functions of welding fluxes
Fluxes perform the following functions:
- physical isolation of the weld pool from the atmosphere;
- arc discharge stabilization;
- chemical interaction with liquid metal;
- weld metal alloying;
- formation of the seam surface.
The best insulating ability is found in fluxes with a dense structure of finely granulated particles. However, when flux particles are densely packed, the formation of the weld surface deteriorates. Sufficiently effective protection of the weld pool from atmospheric influences is provided at a certain thickness of the flux layer.
The required flux layer height for welding low-carbon and low-alloy steels in various modes is as follows:
| Welding current, A | 200 — 400 | 600 — 800 | 1000 — 1200 |
| Flux layer height, mm | 25 — 35 | 35 — 40 | 45 — 60 |
Stabilizing elements are introduced into the flux to increase the stability of the arc. The introduction of these elements makes it possible to use alternating current for welding and to vary welding modes more widely.
The chemical composition of the weld metal is formed by the base and electrode metals. The composition of the flux can also lead to changes in the chemical composition of the weld metal. However, these changes are possible, as a rule, only within fractions of a percent. Ceramic fluxes are used to alloy the weld metal.
The forming ability of fluxes is determined by the viscosity of the slag, the nature of its dependence on temperature, interfacial tension at the metal-slag interface, etc. The forming ability largely depends on the arc power. When welding with a powerful arc (current over 1000 A), good formation is ensured by “long” fluxes, the viscosity of which monotonically decreases with increasing temperature. When welding circumferential welds of small diameter, to prevent slag swelling, “short” fluxes should be used, the viscosity of which sharply decreases with increasing temperature.
The gas permeability of the flux, which is determined by the particle size and bulk mass of the flux, has a significant influence on the formation of the weld. The recommended particle sizes of glassy flux depending on the arc power to ensure satisfactory weld formation are given below.
| Welding current, A | 200 — 600 | 600 — 1200 |
| Particle granulation, mm | 0,25 – 1,6 | 0,4 – 2,5 |
Classification of fluxes
Fluxes can be classified according to:
- manufacturing method;
- chemical composition;
- structure and size of particles;
- purpose.
According to the manufacturing method, fluxes are divided into:
- fused;
- ceramic;
- mechanical mixtures.
Fused fluxes are produced by fusing the components of the charge in electric or flame furnaces.
Ceramic fluxes are made from mixtures of powdered materials held together with adhesives, mainly liquid glass. Sintered fluxes are produced by sintering the components of the charge at elevated temperatures without fusing them. The resulting lumps are then crushed to the required size.
Flux mixtures are made by mechanically mixing grains of various materials or fluxes. A big disadvantage of mechanical mixtures is their tendency to separate into components during transportation and during the welding process due to differences in density, shape and size of the grains. Therefore, mechanical mixtures do not have constant compositions and welding properties and do not reliably provide stable quality welds.
Depending on the chemical composition, fluxes are classified according to their content:
- silicon;
- manganese
Low-silicon fluxes contain less than 35% silicon oxide (SiO2). When the content is more than 1% manganese oxide (MnO), the flux is called manganese. High-silicon fluxes contain more than 35% SiO2; Manganese-free fluxes contain less than 1% MnO. Oxygen-free fluxes occupy a special group when classifying fluxes by chemical composition.
according to the degree of alloying :
- passive (practically no alloying of the weld metal);
- lightly alloyed (fused);
- and alloying (ceramic).
Based on the particle structure, fused fluxes are divided into:
- glassy (transparent grains)
- pumice-like (grains of foamy material white or light shades of yellow, green, brown and other colors).
Pumice-like fluxes have a lower bulk density (0.7-1.0 kg/dm3) than glassy ones (1.1-1.8 kg/dm3). Fused fluxes have found the greatest application.
Depending on the purpose and primary use , fluxes are distinguished for electric arc and electroslag welding, as well as for mechanized welding and surfacing of carbon steels, alloy steels, non-ferrous metals and alloys. This division is to a certain extent arbitrary, since fluxes, primarily used for welding and surfacing of metals or alloys of one group, can be successfully used for welding and surfacing of metals of another group. At the same time, fluxes intended for welding some non-ferrous metals or some grades of alloy steels may be unsuitable for welding other non-ferrous metals or other grades of alloy steels.
General requirements for flux
Fluxes for mechanized welding must ensure a stable welding process, the absence of crystallization cracks and pores in the weld metal, the required mechanical properties of the weld metal and the welded joint as a whole, good formation of the weld, easy separation of the slag crust, minimal release of toxic gases during welding, and also have low cost and the possibility of mass industrial production.
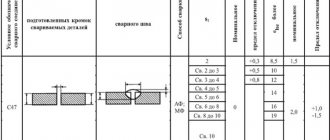
According to EN 760, welding fluxes are classified according to their chemical composition as shown in the table below.
Classification (types) of fluxes by chemical composition
| Symbol | Main components | Flux type | Basicity index |
| MS | MnO + SiO2 > 60%; CaO 15%; ZrO2 | Manganese silicate | 0,8 |
| C.S. | CaO + MgO + SiO2 > 60%; CaO > 15% | Calcium silicate | 0,7-1,2 |
| AR | Al2O3 + TiO2 > 45% | Aluminate-rutile | 0,7-1,4 |
| AB | Al2O3 + CaO + MgO + CaF2 > 55%; Al2O3 > 20%; CaF2 (total fluorine content) 20% | Aluminate-basic | 1,0-2,0 |
| FB | CaO + MgO + MnO + CaF2 > 50%; SiO 2 20%; CaF2 (total fluorine content) > 15% | Fluorite-basic | > 2,0 |
| W | Fluxes whose composition does not fall under any of the specified types | Others |
Flux-wire combinations in submerged arc welding
If the welding and technological characteristics of the submerged arc welding process are determined mainly by the properties of the flux, then the mechanical properties of the metal of the seams and welded joints depend on the flux-wire combinations.
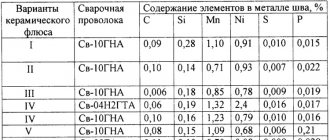
Obtaining high-quality welds on carbon and some low-alloy structural steels is achieved by using the following combinations of fluxes and welding wires: fused high-silicon manganese flux and low-carbon or manganese welding wire, fused high-silicon manganese-free flux and manganese welding wire, ceramic flux and low-carbon or manganese zinc wire.
When using fused high-silicon manganese flux and low-carbon or manganese welding wire or fused high-silicon manganese-free flux and manganese welding wire, the latter must be made of boiling or semi-quiet steel. Calming the metal of the weld pool and preventing porosity when welding boiling steel is carried out as a result of introducing a certain amount of silicon from the flux into the welding zone. Alloying of the weld metal with manganese in order to increase its resistance to the formation of crystallization cracks is done through flux (first and third combinations) or through wire (second and third combinations).
The welding properties of high-silicon manganese fluxes are somewhat better than those of high-silicon manganese-free fluxes. A positive characteristic of high-silicon manganese fluxes is the high resistance of welds against the formation of crystallization cracks. This is due to the small transfer of sulfur from fluxes of this type into the weld metal and the relatively strong burnout of carbon from the metal of the weld pool. In addition, the quality of the weld is positively influenced by the lower carbon content in low-carbon wire used in combination with high-silicon manganese fluxes compared to manganese wire. When welding under them, the porosity of the welds is less than when welding under high-silicon manganese-free fluxes.
If the strength and chemical composition of the weld metal are determined by the chemical compositions of the welding wire and the base metal, then its impact strength largely depends on the flux. High impact strength of the weld metal is ensured by its fine-crystalline structure and low content of inevitable harmful impurities and non-metallic inclusions. To meet these requirements, the SiO2 content in the flux is usually reduced. Therefore, when welding low-alloy steels, low-silicon fluxes are predominantly used. An additional requirement is the lowest possible hydrogen content in the weld metal. Refinement of the structure of the weld metal is also facilitated by a decrease in the heat input of welding. However, this reduces the efficiency of the welding process due to an increase in the number of passes.
In the welding process of modern low-alloy high-strength steels, only limited heat input is allowed to avoid damage to the structure of the base metal in the heat-affected zone. This requirement is met by applying multilayer seams when welding metal of medium and large thickness. In this regard, fluxes intended for welding such steels must ensure easy separation of the slag crust, high quality of weld formation and its mechanical properties. By increasing the mechanical properties of the weld metal by using the appropriate combination of flux and wire, the need for uneconomical thin welds in multi-pass welding of thick metal is eliminated.
Slag-metal and gas-metal reactions, reduction and burnout of elements
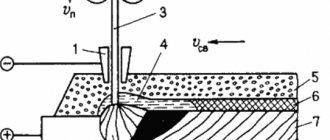
During fusion welding, interaction occurs between the liquid slag and the metal. The duration of this interaction is usually very short. In electric arc welding, it ranges from 10 s to 1 min. The interaction stops after the metal and slag have solidified. Despite the short duration, the interaction reactions between slag and metal during electric arc welding can occur very vigorously, which is due to the high heating temperature of the metal and slag, the large surfaces of their contact and the relatively large relative amount of slag.
The interaction between slag and metal is described by reactions of displacement of one element from slag into metal by another or distribution between slag and metal. Displacement reactions predominantly lead to the enrichment or depletion of the weld metal with alloying elements, while distribution reactions lead to the formation of non-metallic inclusions in the weld metal.
During displacement reactions, metal atoms and slag oxide molecules interact on the contact surfaces of liquid metal and slag. A very significant role is played by the reduction reactions of silicon and manganese:
(MnO) + [Fe] = (FeO) + [Mn]; (SiO2) + 2 [Fe] = 2 (FeO) + [Si].
Symbols in round brackets indicate elements and compounds found in the slag, and symbols in square brackets in the metal. At high temperatures, reactions predominantly proceed from left to right (reduction of manganese and silicon from slag to metal), with decreasing temperature - from right to left (oxidation of manganese and silicon and their transition from metal to slag). The direction of reactions also depends on the concentration of reactants. If the metal of the weld pool contains little manganese and silicon, and the slag contains a lot of MnO and SiO2 and little FeO, manganese and silicon are reduced from the slag to the metal at high temperatures (near the arc). If there is a lot of manganese and silicon in the metal of the weld pool, and there is no MnO and SiO2 in the slag, or a lot of FeO, manganese and silicon are oxidized even in the high temperature zone of the weld pool.
The interaction reactions between the slag and the metal of the weld pool take place under conditions of rapid temperature changes and constant updating of the composition of the reacting phases. In this regard, both the intensity of these reactions and their direction change. However, although the interaction of slag and metal during welding does not reach a state of equilibrium, it is always directed towards its establishment.
The intensity of interaction between slag and metal depends on the welding mode, and it is most strongly influenced by current and arc voltage; Current density and welding speed have little effect. A decrease in current and an increase in arc voltage enhance the interaction of slag and metal, increase the intensity of reduction or oxidation of silicon and manganese during welding, and enhance the transition of sulfur and phosphorus from slag to metal or from metal to slag. In automatic submerged arc welding, the specified mode is maintained constant, certain amounts of electrode and base metals are melted per unit time, and the processes of interaction between the metal, slag and gas phases at high temperatures proceed in the same way. Thanks to the consistency of the automatic welding mode, a seam of a stable chemical composition is obtained. If the chemical composition of the base metal and the welding or filler wire is known, as well as the nature of the change in the chemical composition of the metal of the weld pool as a result of interaction with the slag or gas phase, then it is possible to approximately calculate in advance the chemical composition of the weld that will be obtained when welding in the selected mode.
Classification of fluxes
The most competent approach to studying the types of fluxes and the intricacies of their applications would be to become familiar with GOST 8713 79 on submerged arc welding. This standard is almost forty years old, it has stood the test of time and still works great: it contains everything a professional welder needs to know about this technology.
We highly recommend this GOST. In the meantime, let's look at the classification.
There are many varieties of granular mixtures, they differ according to the following criteria:
By granule size and appearance
Fluxes are divided according to the size of the granules into the following categories:
- granular and crystalline;
- powdery;
- in the form of a paste;
- gaseous.
Powdered flux mixtures are best suited for surfacing or electric welding, while paste or gaseous mixtures are the best option for soldering or gas welding.
The structure of grains or granules can be:
- glassy;
- premzoid;
- cemented.
By chemical composition
Components and types of fluxes.
The chemical composition primarily determines the inertness of mixtures when exposed to high temperatures. In addition, there are mixtures that give the effect of active diffusion of individual elements into the metal of the forming weld.
With all the diversity in the chemical composition and mechanical properties of flux mixtures, there are two elements that are always and without fail present in fluxes: silica and manganese. In addition to them there are various kinds of additives in the form of metals or ferroalloys for alloying.
Given the constant presence of silica and manganese in the composition, the proportion and variety of other additives can vary greatly. Depending on them, fluxes are divided into three groups:
Oxide flux mixtures
They are used in welding fluorine and low-alloy steel alloys. They contain metal oxides and a fairly high proportion of fluorine compounds - up to 10%. Depending on the amount of silicon, oxide fluxes are silicon-free if the proportion of silica in them is less than 5%; low-silicon with a silicon content ranging from 6 to 35% and high-silicon with a silica content of over 35%.
In the same way, oxide fluxes are divided based on the manganese content in them: manganese-free with a manganese fraction of less than 1%; low manganese with a share in the mixture within 10%; medium-high manganese with a percentage of the element from 10% to 30%.
Mixed fluxes
These mixtures contain much less oxides, but more various salts. The proportion of silica is quite low: 15 - 30%, manganese is contained within 9%, but the level of fluorine compounds is increased: the CaF2 content, for example, is increased to 12 - 30%. Mixed fluxes are used in work with alloy steels.
Salt flux mixtures
They have no oxides at all. On the contrary, the content of salt compounds of chlorine and fluorine with calcium, sodium and barium determines the properties and functions of these mixtures. First of all, they are designed for welding chemically active metals. They are also used for smelting.
Suitable for working with all types of steel alloys: carbon and alloy. Non-ferrous metals are also included in the range of acceptable elements of salt fluxes.
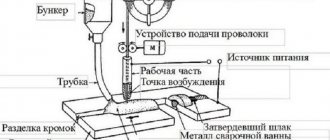
Automatic submerged arc welding modes.
There is another important chemical characteristic of fluxes - its chemical activity. It consists of the final oxidative abilities of the elements. According to this criterion, protective mixtures are divided into several types: from highly active with an activity index of more than 0.6 to passive with an activity index of below 0.1.
According to the method of action of the flux mixture
Fluxes differ in the same way as electrodes: there are melting and non-melting types. Fusible fluxes work great when the metal surface needs additional elements to improve, for example, the appearance or increase the metal's corrosion resistance.
Non-consumable fluxes are used when the main goal is to improve the mechanical properties of the weld. Most often, this type of welding under a layer of flux is found when joining non-ferrous metals, high-carbon steels and aluminum - all of these metals are characterized by capriciousness and complexity of welding.
By purpose
There are, for example, welding fluxes that are specially alloyed to improve the chemical composition and quality of the weld. But the most popular are universal granular mixtures that can be used in working with all types of metals - from high-alloy steel alloys to aluminum and tin in their pure form.
Fluxes for low carbon steels
Only oxide options are used here. They are found with two different combinations of the wire-flux system. The first combination is flux mixtures with high proportions of silicon and manganese together with low-carbon steel wire without any alloying additives.
As a result, the weld is alloyed with manganese from the flux. This combination is used mainly in Russian granular mixtures.
The second combination is a steel welding flux with little or no manganese and a high silicon content combined with low carbon manganese alloyed steel wire. The weld is alloyed with manganese from the wire.
In this case, the submerged arc welding wire becomes the alloying source. This combination is more often used in foreign welding technologies.
Fluxes for low alloy steels
Submerged arc welding diagram.
To work with low-alloy alloys, fluxes with low chemical activity are needed, lower than for low-carbon alloys. This property causes an increase in the ductility of the weld. But at the same time, the formation of pores in the seam increases, and its formation is worse.
Fluxes for high alloy steels
High-alloy alloys mean that a significant amount of various additives have been added to the steel to give additional properties to these alloys. In such cases, it would be logical to use granulated flux mixtures with minimal chemical activity, which contain small proportions of silicon.
As for manganese, it is practically absent in fluxes of this kind.
Fluxes for active metals
An example of an active metal is titanium, which is one of the most difficult metals to weld. Special mixtures consisting entirely of salts have been created for them - the so-called salt fluxes. They do not contain oxides to maintain the plasticity of the seams, because the admixture of oxygen always reduces it.
The main components of salt mixtures are fluoride and chloride salts of fir and alkaline earth metals.
Handling and storage of welding fluxes
To avoid the appearance of pores in the seams, the moisture content of welding fluxes should not exceed established standards. The humidity of AN-60 flux should not exceed 0.05%; for other brands of fused fluxes produced in accordance with GOST 9087-81 no more than 0.10%.
High-humidity fluxes are dried in ovens at 100-110°C (vitreous fluxes) and 290-310°C (pumice-like fluxes). Fluoride fluxes are calcined at 500-900°C.
When fluxes are reused, their particle sizes decrease. Therefore, you should periodically sift the flux through a sieve and perform submerged arc welding at lower welding currents.
Electrodes and welding wire with flux
This electrode manufacturing technology has greatly facilitated the work of welders. The layer of flux that is applied to the welding wire or electrodes performs the same tasks as the flux applied to the surfaces of the parts.
But the welder has complete control over the weld during the work process.
When the electrode is heated, the flux melts, flows off its surface and covers the edges of the parts. Its protective functions remain the same. Another feature of flux electrodes is the release of carbon dioxide from the molten flux. It is known that carbon dioxide does not support combustion. Consequently, it is possible to increase the welding current without fear of overheating of parts.