Features of the steel production process
In the production of cast iron and steel, different technologies are used, despite the fairly similar chemical composition and some physical and mechanical properties. The differences are that steel contains less harmful impurities and carbon, due to which high performance is achieved. During the smelting process, all impurities and excess carbon, which causes an increase in the fragility of the material, go into slag. Steel production technology involves forced oxidation of basic elements due to the interaction of iron with oxygen.
Melting steel in an electric furnace
When considering the production process of carbon and other types of steel, several main stages of the process should be highlighted:
- Rock melting. The raw materials that are used to produce metal are called charge. At this stage, during the oxidation of iron, impurities are also deoxidized. Much attention is paid to reducing the concentration of harmful impurities, which include phosphorus. To ensure the most suitable conditions for the oxidation of harmful impurities, a relatively low temperature is initially maintained. The formation of iron slag occurs by adding iron ore. After the release of harmful impurities on the surface of the alloy, they are removed, and a new portion of calcium oxide is added.
- Boiling of the resulting mass. After the preliminary stage of cleaning the composition, baths of molten metal are heated to a high temperature, and the alloy begins to boil. Due to boiling, the carbon contained in the composition begins to actively oxidize. As previously noted, cast iron differs from steel in having too high a carbon concentration, due to which the material becomes brittle and acquires other properties. This problem can be solved by injecting pure oxygen, due to which the oxidation process will occur at high speed. When boiling, bubbles of carbon monoxide are formed, to which other impurities also adhere, due to which the composition is purified. At this stage of production, sulfur, which is a harmful impurity, is removed from the composition.
- Deoxidation of the composition. On the one hand, adding oxygen to the composition ensures the removal of harmful impurities, on the other hand, it leads to a deterioration in basic performance qualities. That is why, to clean the composition from harmful impurities, diffusion deoxidation is often carried out, which is based on the introduction of a special molten metal. This material contains substances that have approximately the same effect on the molten alloy as oxygen.
In addition, depending on the characteristics of the technology used, two types of materials can be obtained:
- Calm ones who have gone through the deoxidation process to the end.
- Semi-quiet, which have a state between calm and boiling steels.
During the production of the material, pure metals and ferroalloys can be added to the composition. Due to this, alloyed compounds are obtained that have their own specific properties.
This is interesting: High-speed tool steels: grades, characteristics, markings
Electrothermal method
Today, electrothermal steel production is considered the most effective. Thus, in comparison with open-hearth furnaces and converters, this technique provides the possibility of more accurate control of steel quality, including through the regulation of the chemical composition. The interaction of furnace chambers with the air environment also deserves special attention. Electrothermal steel production technology provides for minimal access to air, leading to other advantages. For example, this allows minimizing the accumulation of iron monoxide and foreign particles in the alloy, as well as ensuring more efficient burnout of phosphorus and sulfur.
The high temperature regime at 1650 °C makes it possible to melt problematic slags that require thermal exposure at increased power. Also in electric furnaces it is possible to alloy steel with refractory metals, including tungsten and molybdenum. However, this method of producing steel also has a serious drawback. The ovens used require large amounts of energy, making this process the most expensive.
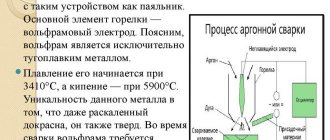
Argon arc welding
In principle, what was said above fully applies to argon arc welding with a non-consumable electrode in a shielding gas environment. The only thing added to welding technology is additive and gas. Everything is clear with gas, because it is the same for all types of welding, regardless of the type and type of workpieces being joined, or rather, their metals.
But the filler material is selected according to the properties of the base metal being welded. And since there are two of them, you will have to take into account the welding abilities of each of the two. And since cast iron is, so to speak, weak, then the additive should be selected according to it. So, as a filler material, you can use welding flux-cored wire, from which the coating is beaten off. Some craftsmen use strips of cast iron, cut into small pieces.
True, both materials can be used only if the welder has enough experience in using this technology. The thing is that an incorrectly set mode and an incorrect melting speed of the additive can lead to the formation of so-called intermetallic structures in the weld pool. They have increased fragility. This is why practical experience is considered an important component of the quality of the final result when welding steel and cast iron with a non-consumable tungsten electrode.
Therefore, some purely practical advice.
- To weld two metals, it is best to use nickel-based additives, which were specifically invented for welding cast iron products. But if the requirements for the strength and reliability of the joint are quite large, then even such an additive will not be able to fulfill them.
- You cannot cook cast iron with steel with a wide bath and large heat inputs.
- It is recommended to deposit a nickel additive on the cast iron edge before starting welding work. That is, to form the so-called transition layer.
- You can weld cast iron to steel without preheating or with it. The first option is chosen only if during operation of the welded part it will not be subjected to great heat, no more than +300C. If there are no such requirements, then heating will have to be done. Small workpieces are heated completely, large ones only in the welding zone.
- Cast iron is a metal with low ductility and very low linear expansion compared to steel. Therefore, it is very important to reduce shrinkage stresses in the weld. Cast iron shrinks a little, steel, on the contrary, shrinks a lot.
What is steel
Steel is an iron-carbon alloy in which the carbon content does not exceed 2.14%. An alloy containing more than 2.14% carbon is called cast iron.
In turn, steels are divided into: low-carbon (up to 0.6% carbon) and high-carbon (0.6-2.14%).
The higher the carbon content in an alloy, the harder and stronger the alloy is, but less viscous and ductile.
Converter method
With this method, molten cast iron can be used as a base, as well as impurities and waste in the form of ore, scrap metal and flux. Compressed air is supplied through technological holes to the prepared base, facilitating chemical reactions. The process also involves thermal action, which causes the oxidation of oxygen and impurities. The characteristics of the furnace structure in which the steel is processed are also of particular importance. Steel can be produced in units with different linings - the most common methods of protecting structures are fire-resistant bricks and dolomite mass. Based on the type of lining, the converter method is also divided into two other methods: Thomas and Bessemer.
Applications of argon arc welding
The use of refractory electrodes and copper-nickel filler wire gives good results.
How to weld steel to cast iron (several recommendations):
- The additive is selected according to the type of metal and type of cast iron. Nickel-based flux-cored wire is most often chosen; the coating is first removed from it.
- Argon is used as a protective atmosphere; it saves the seam from oxidation.
- Refractory tungsten electrodes are chosen for work.
- To work with thin-walled elements, experienced welders use small thin pieces of cast iron instead of wire.
- For connections that experience only static loads (compression), nickel alloys can be used. They adhere perfectly to any grade of steel.
After-furnace processing
Steel for critical products is produced with mandatory out-of-furnace treatment, including evacuation.
The decisive factors for the efficiency and productivity of secondary processing processes are high availability, short charging cycles, low consumption rates, as well as reduced labor costs. An important factor in the quality implementation of out-of-furnace processing technology is:
- finely tuned automatic process control system, ensuring efficient use of personnel and reduction of the production cycle;
- precise determination of design parameters for the materials used can guarantee verifiable compliance with consumption indicators;
- the correct choice of unit design leads to a reduction to a minimum cost of the areas required to accommodate a vacuum pump, alloying supply system and vacuum chambers
At the world's leading enterprises in the production of metal for the needs of mechanical engineering, evacuation is carried out using both ladle (VD), batch (DH) and circulation (RH) evacuation units. The most common are ladle degassers, since it is degassers of this type that allow for intensive mixing of metal with slag, thereby carrying out deep desulfurization.
This is interesting: Steel grades - table with markings and interpretation
How to weld cast iron to metal using electric welding
When carrying out repair work, situations arise when it is necessary to connect dissimilar metals. Is it possible to weld cast iron and steel? Yes, but this can be done by people with experience. Cast iron alloys are characterized by a high carbon content; when it burns out, the structure of the metal changes. The joint is boiled at low temperature, up to 120°C. Steel, on the contrary, needs to be very hot. A buffer layer technology has been developed to connect cast iron parts with other ferrous alloys. Nickel alloy provides a strong connection between dissimilar metals.
Steel processing techniques
The process of final formation of the metal structure is not always completed after the main production. In the future, in order to improve the characteristics of the product, additional processing tools can be used. These include deformation methods in the form of forging, stamping and rolling. This helps, already at the production stage, to form a set of necessary technical properties that the finished steel will have. The output of steel produces a plastic structure, which is why primary processing technologies are quite diverse. Thus, in addition to deformation, methods of hardening, annealing and normalization can be used.
Necessary equipment
Steel production technology involves the use of the following equipment in steel mills.
Oxygen converter section:
» data-lazy-type=»iframe» src=»data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7″>
- argon supply systems;
- converter vessels and their supporting rings;
- dust filtration equipment;
- system for removing converter gas.
Electric furnace section:
- induction furnaces;
- arc furnaces;
- containers used for loading;
- scrap metal storage area;
- converters designed to provide induction heating.
Secondary metallurgy site where:
- cleaning steel from sulfur;
- steel homogenization;
- electroslag remelting;
- creation of a vacuum environment.
Boiling steel
Area for implementation of bucket technology:
- LF equipment;
- SL equipment.
The bucket facility providing steel production also includes:
- bucket covers;
- casting and pouring ladles;
- gate valves.
Steel production also requires equipment for continuous casting of steel. Such equipment includes:
- rotating frame for manipulating pouring ladles;
- equipment for continuous casting;
- trolleys on which intermediate buckets are transported;
- trays and vessels intended for emergency situations;
- tundishes and storage areas;
- plug mechanism;
- mobile mixers for cast iron;
- cooling equipment;
- areas where continuous casting is performed;
- internal rail-type vehicles.
The production of steel and the manufacture of products from it is a complex process that combines chemical and technological principles, a whole list of specialized operations that are used to produce high-quality metal and various products from it.
» data-lazy-type=»iframe» src=»data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7″>
Article rating:
Second phase
The stage begins as the metal bath is heated to higher temperatures than in the first stage. As the temperature rises, the carbon oxidation reaction occurs more intensely, taking place with the absorption of heat. To oxidize carbon at this stage, a significant amount of ore, scale, or oxygen is injected into the metal.
The iron oxide formed in the metal reacts with carbon and bubbles of carbon monoxide CO are released from the liquid metal, causing the bath to boil. When the bath is boiling:
- the carbon content in the metal decreases;
- the temperature and composition of the bath are equalized;
- Non-metallic inclusions in the slag are partially removed.
- All this helps to improve the quality of the metal.
During the same period, conditions are created for removing sulfur from the metal. Sulfur in the bath is in the form of iron sulfide dissolved in metal and slag (FeS). The higher the temperature, the more FeS is dissolved in the slag or the more sulfur is transferred from the metal to the slag. Iron sulfide, dissolved in the slag, reacts with calcium oxide CaO, also dissolved in the slag, forming the compound CaS, which is soluble in the slag, but not soluble in the metal. In this way, the sulfur is removed into the slag.
Thomas method
A special feature of this method is the thorough processing of cast iron containing up to 2% phosphorus impurities. As for the lining technique, it is implemented using calcium and magnesium oxides. Thanks to this solution, slag-forming elements are endowed with an excess amount of oxides. The phosphorus combustion process is one of the key sources of thermal energy in this case. By the way, the combustion of 1% phosphorus filling increases the temperature of the furnace by 150 °C. Thomas alloys have a low carbon content and are most often used as technical iron. In the future, wire, roofing iron, etc. are made from it. In addition, the production of steel (cast iron) can be used to produce phosphorous slag for further use as a fertilizer on soils with high acidity.
Third stage
This stage is the final stage, in which the steel is deoxidized and, if required, alloyed. Deoxidation is a technological operation in which oxygen dissolved in a metal is converted into an insoluble compound and removed from the metal. During smelting, an increased oxygen content in the metal is necessary for the oxidation of impurities. In finished steel, oxygen is an undesirable impurity, since it reduces the mechanical properties of steel, especially at high temperatures.
To deoxidize steel, deoxidizing elements are used that have a greater affinity for oxygen than iron. Manganese, silicon, and aluminum are used as deoxidizers. There are several ways to deoxidize steel. The most widely used:
- precipitating method;
- diffusion.
Siege method
Deoxidation using this method is carried out by introducing deoxidizing agents (ferromanganese, ferrosilicon, aluminum) containing Mn, Si, Al into liquid steel. As a result of deoxidation, oxides MnO, SiO2, Al2O3 are formed, which have a lower density than steel and are removed into slag. However, some of the oxides do not have time to float and are removed from the metal, which reduces its properties. This method is sometimes called deep, since deoxidizing agents are introduced into the depth of the metal.
Diffusion method
In this method, deoxidation is carried out by deoxidizing the slag. Ferromanganese, ferrosilicon and other deoxidizers are loaded in finely ground form onto the slag surface. Deoxidizers, by reducing iron oxide, reduce its content in the slag. In accordance with the distribution law, iron oxide dissolved in steel will begin to turn into slag. The oxides formed during this deoxidation method remain in the slag, and the reduced iron goes into steel, which reduces the content of non-metallic inclusions in it and improves its quality.
Due to the fact that the speed of the process of moving oxygen from the metal to the slag is determined by the speed of its diffusion in the metal, this method also has some disadvantages. Due to the low rate of oxygen diffusion in the metal, the process of oxygen removal is slow, and the duration of smelting increases. Depending on the degree of deoxidation, steels are distinguished:
- boiling;
- calm;
- semi-calm.
Boiling steel
This is steel smelted without deoxidation. When casting such steel and during its gradual cooling in the mold, a reaction will occur between oxygen and carbon +=COg dissolved in the metal
The resulting bubbles of carbon monoxide CO will be released from the crystallizing ingot, and the metal will seethe. This type of steel is called boiling steel. Boiling steel contains practically no non-metallic inclusions representing deoxidation products. Therefore, it has good ductility.
Calm steel
This is steel obtained after a deoxidation operation. When solidified in a mold, such steel behaves calmly and does not emit gases. This type of steel is called calm steel. Semi-quiet steel. Steel has an intermediate deoxidation between calm and boiling. Its deoxidation is carried out partially, removing not all oxygen from it. The remaining oxygen causes a brief boiling of the metal at the beginning of its crystallization. This type of steel is called semi-quiet.
Alloy steels
Alloying is the process of adding special (alloying) elements to steel in order to obtain so-called alloy steel with special physical, chemical or mechanical properties. Alloying is carried out by introducing ferroalloys or pure metals in the required quantity into the alloy. Alloying elements whose affinity for oxygen is less than that of iron (Ni, Cu, Co, Mo) practically do not oxidize during melting and casting and therefore they are introduced into the furnace at any time during melting. Alloying elements whose affinity for oxygen is greater than that of iron (Si, Mn, Al, etc.) are introduced into the metal after or simultaneously with deoxidation.
SHARE INTERESTING INFORMATION
Technology for producing quality steel
5. PROCESSING OF METAL WITH SYNTHETIC SLAG
Mixing the metal with specially prepared (“synthetic”) slag makes it possible to intensify the process of transition into the slag of those harmful impurities that are removed into the slag phase (sulfur, phosphorus, oxygen). In cases where the main role in removing impurities belongs to the slag phase, the rate of the process is proportional to the size of the interphase surface area. Most often this method is used to remove sulfur, so the main component of synthetic slag is lime (sometimes also fluorspar); Since there are practically no iron oxides in such tar, it is at the same time a good deoxidizing agent. In cases where the task is to clean the metal from non-metallic inclusions of a certain composition, the composition of the synthetic slag is selected accordingly. In practice, the task is, firstly, to obtain slag of the desired composition and temperature and, secondly, to develop a method for obtaining the maximum contact surface of the slag and metal phases. Naturally, the conditions necessary for the subsequent separation of slag from the metal must be provided.
The treatment of steel in a ladle with liquid synthetic slag, as a method of purifying the metal from unwanted impurities, was proposed in 1925 by the Soviet engineer A.S. Tochinsky; in 1933, a method for processing metal with liquid lime-alumina slag was patented by the French engineer R. Perrin.
Among the tested methods of processing metal with slag, the following are widely used:
a) processing of steel during its production with solid mixtures (usually consisting of CaO and CaF2) melted due to the heat of liquid steel for the purpose of desulfurization;
b) treatment of steel with liquid lime-alumina slag for the purpose of desulfurization and deoxidation of the metal;
c) processing the metal during casting and crystallization with slags of various compositions in order to remove harmful impurities and obtain a good surface of the ingot;
d) introducing synthetic solid mixtures (consisting of CaO, SiO2 and Al2O3) onto the liquid metal in the ladle, melting them due to the heat of arcs from electrodes introduced through the ladle lid, and blowing the metal from below with argon (or nitrogen) to mix with the slag for the purpose of desulfurization and removal of non-metallic impurities.
In 1927, A.S. Tochinsky, for the first time in the world, conducted industrial experiments on the dephosphorization of Bessemer steel with lime-iron slag, and in 1928-1929. refined basic open-hearth steel with acidic slag for deoxidation (the oxygen content in the metal was reduced by 30-55%). Later, lime-iron slag (60-65% CaO and 20-35% iron oxides) was repeatedly used to process converter steel, obtaining a high degree of dephosphorization. Thus, the phosphorus content in Thomas steel was reduced from 0.060 to 0.010%, and in Bessemer rail steel from 0.05-0.09 to 0.01-0.03%. Experience has shown, however, that treatment of carbon metal with calc-ferrous slag leads to violent boiling and emissions. In addition, treatment with ferrous slag made it difficult to carry out the metal deoxidation operation.
As for the method of processing steel with lime-alumina slag, in the USSR the corresponding research work was widely launched, starting in 1959 at the Central Scientific Research Institute of Ferrous Metallurgy (TSNITSChM) and at a number of factories. In the process of experimental work, the optimal compositions of slags for various grades of steel were determined and units were designed for melting them and feeding them to the steel-smelting unit. According to the TsNIICHM technology, tars with a high content of CaO and Al2O3 additives (to reduce their melting temperature and ensure the necessary fluidity) are melted in a special electric furnace and poured into a steel-pouring ladle when releasing steel from a steel-smelting furnace or from a converter. When pouring the metal onto the synthetic slag in the ladle, both reacting phases (steel and slag) are intensively mixed, the slag emulsifies in the metal and, to some extent, emulsifies the metal in the slag, followed by phase separation. The intensity and depth of the process are determined by the height of the drop of the metal jet, the mass of the metal and putty, the physical characteristics and composition of the slag, etc. The task is to ensure the maximum value of the interphase surface during the processing. The greatest influence in this case is the height of the fall of the metal jet, as well as the viscosity of the slag.
A variation of the method of processing steel with liquid synthetic slag is the so-called mixing method, when steel, synthetic slag, and liquid master alloy (molten ferroalloys) are simultaneously mixed in a steel-pouring ladle. In the USSR, the method of mixing a semi-product with a liquid master alloy in a steel-pouring ladle with simultaneous processing with synthetic slag is used to produce high-quality ball-bearing steel. Intermediate composition ~ 0.35% C; ~0.10% Mn and traces of Si are smelted in an open hearth furnace. Ligature composition - 3.2% C; 0.6-2.0% Mn; 1.3-3.4% Si and 6.0-6.5% Cr are obtained in an electric arc furnace. The mass ratio of the intermediate product and alloy is ~4:1.
The low-carbon intermediate product, which contains practically no manganese and silicon, is overoxidized in relation to the composition of the alloy. Therefore, at the moment of mixing, the intermediate product is rapidly deoxidized by the carbon contained in the alloy, with the formation of gaseous deoxidation products that are completely removed from the melt. All this occurs simultaneously with the influence of synthetic slag poured into the ladle on the melt, which ensures the purification of the metal from sulfur and non-metallic inclusions. As a result, for example, 120 tons of high-quality steel are obtained from 100-ton open-hearth melting and 20-ton heat from chipboard. Sometimes the melting of the master alloy is combined with the melting of synthetic slag, i.e. in one electric furnace both the alloy and the required amount of synthetic slag are melted; The master alloy and slag thus obtained are poured into a ladle, into which the smelting is then released from an open-hearth furnace or converter. This process is called “combined” (Fig. 84).
The mixing method was developed in 1970-1975. at the Izhevsk Metallurgical Plant. It ensures the production of high quality metal. Thus, according to the work [52], the durability of bearings made of ShKh15 steel, smelted using the mixing method, is 2.9 times higher than usual.
Methods such as the mixing method or a combined process make it possible, if necessary, to ensure the production of high-quality steel in an open-hearth or converter shop using relatively simple equipment. What is common in both cases is the use of synthetic slag as an affordable way to reduce the content of sulfur and non-metallic inclusions in steel and correspondingly improve the quality and reliability of the metal. A very important circumstance in this case is also the ability to achieve more standard quality indicators from heat to heat when processing metal with synthetic slag.
The consumption of synthetic slag is relatively small: 3-5% by weight of the metal. With a relatively small amount of slag, it is easier to ensure the standardization of its composition and properties. Therefore, processing steel (with some inevitable fluctuations in composition and properties from heat to heat) with synthetic slag of strictly standard composition and temperature makes it possible to solve the very important task of producing reliable and standard products at a metallurgical plant. The additional costs for producing synthetic slag are justified by the benefits that the national economy receives by using higher quality steel.
Based on the developments of the TsNIICHM, the method of processing metal with synthetic slag became widespread in the USSR. TsNIIChM has developed a group of synthetic slags, mainly consisting of lime and alumina with a silica content of up to 10-15%, which have sufficient fluid mobility at liquid steel temperatures; Continuous slag melting furnaces of the OKB-1320 type have been created, making it possible to smelt slag in quantities sufficient to process 600-800 thousand tons of steel per year at a consumption of 4-5% relative to the mass of metal. In the future, the productivity of these furnaces can be more than doubled [53].
The main requirement for synthetic lime-alumina slags is minimal oxidation (this provides good conditions for deoxidation of steel and its desulfurization) and maximum CaO activity. Therefore, synthetic lime-alumina slags should not contain iron oxides at all, and the silica content should be limited. It is clear that the presence of phosphorus in such slags is excluded, since during processing it will turn into metal. In cases where the charge from which the slag is melted contains a certain amount of silica, magnesia is introduced into the slag, which forms magnesium silicates and thus reduces the harmful effects of silica, which reduces the activity of CaO. The usual composition of synthetic slag used in USSR factories is as follows, %: 50-55% CaO; 37-43 Al2O3; up to 7 SiO2 (in some cases up to 10-15 SiO2); up to 7 MgO. The melting temperature of the slag, depending on the composition, varies from ~ 1400 (in the slag 50-55% CaO; 38-43% Al2O3 and <4.0% SiO2) to ~ 1300 0C (in the slag up to 6-7% SiO2 and 6-7 % MgO).
In order for the slag droplets to easily separate and float up, it is necessary to select slags whose interfacial tension at the boundary with the metal after finishing the metal processing would be maximum. Practice has shown that the total content of non-metallic inclusions after treatment with synthetic slag is reduced by approximately half.
The great advantage of such a technological method as processing steel with synthetic slag is its short duration. The entire operation is completely carried out during the release (draining) of metal from the unit into the ladle; the productivity of the units increases, since technological operations such as desulfurization and deoxidation are transferred to the ladle.
When carrying out the operation of processing metal with slag, it is necessary to take into account the undesirability of slag from the furnace or from the converter getting into the ladle in which the processing is carried out, along with the metal, meanwhile, the task of cutting off the slag when releasing metal from the converter or open-hearth furnace is practically very difficult.
The treatment itself with synthetic slag makes it possible to somewhat reduce the oxidation of the metal, but not so much as to completely abandon the use of deoxidizers. Therefore, in addition to the slag, the required amount of deoxidizing agents is introduced into the ladle. Considering the low density of ferrosilicon, the required amount is loaded onto the bottom of the ladle even before synthetic slag is poured into the ladle. After the melt is released, materials such as ferromanganese and ferrochrome are added to the stream of steel falling into the ladle. Following this, alloys containing titanium, vanadium, zirconium, etc. are added. Aluminum is introduced into the depths of the ladle on rods after the end of the melt tapping.
During the process of mixing metal with slag, the composition of the slag undergoes certain changes; These changes are related to the following:
a) when stirring, the slag interacts with the ladle lining, part of the lining turns into slag;
b) sulfur is removed from the metal and goes into slag;
c) deoxidizers introduced into the ladle are partially oxidized, the resulting oxides pass into slag;
d) some part of the final slag usually ends up in the ladle; The iron oxides contained in the final slag hinder the deoxidation processes. It is especially dangerous if final slag gets into the ladle because of the phosphorus it contains: during the deoxidation process, almost all the phosphorus contained in the final slag is reduced and goes into the metal.
The ''dilution'' of synthetic slag as a result of all these processes can reach 30-40%. However, even in the case when a certain proportion of the final slag does end up in the ladle, the effect of the synthetic slag on the metal entering the ladle, even during those few minutes during which production continues, turns out to be positive and provides a noticeable improvement in the composition and properties of the metal. And in this case, the simultaneous effect of both slag and deoxidizers is useful.
An example of a technology where furnace slag enters a ladle and “dilutes” the synthetic slag poured into it is the technology developed by the Central Research Institute of Chronology and Mechanics and OXMK [54] for 400-ton open-hearth furnaces operating in the scrap-ore process. Synthetic slag from the transfer ladle is poured into steel-pouring ladles installed under a bifurcated chute during the release of the melt into a stream of metal. The slag begins to be drained after releasing part of the metal into the ladle (5-20%) and continues draining for 20-40 s. The end of the slag filling operation corresponds to filling the ladle with metal to no more than one third. The maximum amount of ferroalloys is introduced into the ladle. After the metal is released, the furnace slag is not cut off, but is passed through steel-pouring ladles. The consumption of synthetic slag is 17-22 kg/t when smelting steels of type 10HSND, 15HSND and up to 53 kg/t for steel 12GN2MFAYU. The sulfur content of the finished steel is (on average) 0.015% and <0.012%, respectively.
Practice has shown that when treating steel with a lower carbon content (which, as a rule, has higher oxidation) with slag, the refining effect of oxygen is more pronounced. Accordingly, the consumption of deoxidizers is reduced. Experience has shown that refining low-alloy sheet structural steel with a low carbon content, smelted in 400 open-hearth furnaces, with lime-alumina slag allows one to stabilize the waste of deoxidizers and alloying agents and more reliably obtain the required chemical composition, and a significant increase in the impact strength of steel allows one to expand the production of structural steel. metal intended for use in the northern regions of the country, and obtain an economic effect from the use of metal products of improved quality.
It should be borne in mind that the method of treating metal with synthetic slag provides standard desulfurization results to known limits (usually no more than 0.005-0.007%). In cases where it is necessary to sustainably obtain lower sulfur concentrations, other methods are used, for example, treatment with 1 Whole or rare earth metals. For example, in the conditions of the Novolipetsk Metallurgical Plant (NLMZ), when producing steel for large-diameter pipes in the northern version, refining with synthetic slag alone was not enough to obtain the required impact strength of steel at low temperatures. To do this, it turned out to be necessary to reduce the sulfur content to 0.003-0.005%, which was achieved
This is due to the creation of an integrated technology for processing metal with synthetic slag with a cut-off at the outlet of converter slag and subsequent processing of the metal with argon with the simultaneous injection of powdered calcium-containing materials or rare earth metals. Along with the required reduction in sulfur content, this technology also reduced (about three times) the overall contamination of the metal with inclusions of all types; inclusions mainly take a globular shape [53].
The widespread use of the method of processing metal with synthetic slag at factories in the USSR is due to a number of circumstances. A certain role is played by the fact that in a significant part of the country the operating conditions for metal products in winter become significantly more difficult. To increase the viscosity properties and cold resistance of steel, a reduction in sulfur content is required. Reducing the sulfur content not only increases the toughness properties of the metal, but also significantly improves its weldability, which is especially important when operating welded structures.
Technology of welding cast iron with steel using a consumable electrode
There are several methods of welding cast iron; they are used to join cast iron alloys with other metals:
- Hot - before joining the parts, the ovens are preheated to 600°C completely. Heating with a blowtorch is allowed only in the work area (cast iron becomes crimson in color). Used for reliable connections.
- Semi-hot, good for alloys. The parts to be joined are partially or completely heated to 200–300°C.
- Cold - parts are connected without heating, used for overlays - seams working under compression.
Joining metals by manual electric welding involves preliminary preparation of surfaces. Cast iron alloy is porous and absorbs oil well. It is not enough to degrease the stain; it must be cleaned to a clean layer, otherwise the seam will turn out loose.
How to weld steel to cast iron:
- First you need to select the current parameters. If the welding machine only works on constant, switch the Polarity to reverse. It is necessary to take into account the no-load indicator (voltage between the poles without an arc), the permissible value is up to 54 V. Operating on alternating current is allowed only with no-load from 54 V.
- Welding is carried out in small sections, the beads are made no more than 3 cm long. First, cast iron is deposited, then a steel part is welded to the buffer layer.
- Thick-walled seams are welded in multi-layers, each one is hammered before applying the next. It is made perpendicular to the first - this is how the strength of the connection is achieved.
- After joining the cast iron alloy with steel using hot and semi-hot methods, it is important to observe the cooling regime of the surfacing zone. The seam is periodically heated so that it cools gradually. When suddenly cooled, the cast iron alloy cracks due to internal stresses.
Open hearth method
This method is used to produce high-quality steels used in critical machine parts and precision mechanisms.
At one time, it replaced the labor-intensive and low-productivity crucible and pulding melts that had been used previously.
The loading capacity of one reverberatory furnace used in this method reaches 500 tons. A special feature of the open-hearth method is the ability to remelt not only pig iron, but also metallurgical waste and scrap metal.
The heating temperature of liquid steel reaches 2 thousand degrees. This result is achieved by the special design of the open hearth furnace:
- the use of additional heat from regenerators obtained by burning coke oven or blast furnace gas in a stream of hot air;
- reflections from the roof of the injected gas as a result of fuel combustion in it occurring above a bath of metal, which contributes to the rapid heating of the contents;
- using reversal of the heating flow.
An open hearth furnace consists of the following elements:
- working space with fire-resistant lining of walls and filling windows;
- hearths (bases) made of magnesite brick;
- furnace roof;
- furnace heads;
- slag for removing dust;
- regenerator with changeover valves.
The smelting process takes from 4 to 12 hours. In order to speed up the smelting process, the volume of pumped oxygen exceeds the requirements, which increases smelting productivity by 20–30%.
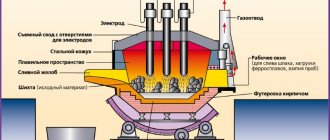
Electric steelmaking method
Steel production by electric melting has a number of undeniable advantages. This method is considered the main one for the smelting of high-quality alloy steels.
The high temperature achieved in this case allows the smelting of steels containing refractory metals:
- molybdenum;
- tungsten;
- vanadium.
High quality is achieved by the virtual absence of phosphorus, sulfur and oxygen in steels. This method is also used to produce a wide range of construction steels.
The release of heat is not associated with the consumption of the oxidizer, but occurs as a result of the conversion of electrical energy into thermal energy. It is released during the passage of an electric arc or the induction of eddy currents. Depending on the principle of operation, furnaces are divided into electric arc and induction.
An electric arc furnace can simultaneously accept from 3.5 to 270 tons of raw materials:
- liquid steel from converters;
- scrap;
- iron ore.
It has several electrodes made of graphite-containing material, to which electrical voltage is applied. The melting time is up to 1.5 hours, while the arc temperature reaches 6 thousand degrees.
Oxygen-converter method of steel production
Steel production today is carried out mainly in this way. BOF production recently accounted for up to 60% of global steel production.
However, this percentage is decreasing due to the advent of electric arc furnaces (EAFs). The furnaces are purged with pure oxygen (99.5%) under high pressure.
The product of an oxygen converter furnace is steel with specified chemical properties. It enters a continuous casting machine (CCM), where the material solidifies into a bloom or slab. To obtain certain stringent parameters, the metal is recycled.