Raw materials for production
What raw materials is aluminum obtained from? Producing aluminum from all the minerals that contain it is expensive and unprofitable. It is mined from bauxite, which contains up to 50% aluminum oxides and lies directly on the surface of the earth in significant masses.
These aluminum ores have a rather complex chemical composition. They contain aluminas in an amount of 30-70% of the total mass, silicas, which can be up to 20%, iron oxide in the range from 2 to 50%, titanium (up to 10%).
Alumina, and this is aluminum oxide, consists of hydroxides, corundum and kaolinite.
Recently, aluminum oxides began to be obtained from nephelines, which also contain oxides of sodium, potassium, silicon, and alunites.
To produce 1 ton of pure aluminum, about two tons of alumina are needed, which, in turn, is obtained from about 4.5 tons of bauxite.
Bauxite deposits
The world's bauxite reserves are limited. There are only seven areas around the globe with its rich deposits. These are Guinea in Africa, Brazil, Venezuela and Suriname in South America, Jamaica in the Caribbean, Australia, India, China, Greece and Turkey in the Mediterranean and Russia.
In countries where there are rich bauxite deposits, aluminum production can also be developed. Russia mines bauxite in the Urals, in the Altai and Krasnoyarsk territories, in one of the regions of the Leningrad region, and nepheline on the Kola Peninsula.
The richest deposits belong to the Russian united company UC RUSAL. It is followed by the giants Rio Tinto (England-Australia), which has teamed up with the Canadian Alcan and CVRD. In fourth place is the Chalco company from China, then the American-Australian corporation Alcoa, which are also large aluminum producers.
Origin of production
Danish physicist Oersted was the first to isolate aluminum in its free form in 1825. The chemical reaction took place with aluminum chloride and potassium amalgam, instead of which two years later the German chemist Wöhler used potassium metal.
Potassium is a fairly expensive material, so in the industrial production of aluminum, the Frenchman Sainte-Clair Deville instead of potassium in 1854 used sodium, a much cheaper element, and stable double chloride of aluminum and sodium.
Russian scientist N. N. Beketov was able to displace aluminum from molten cryolite with magnesium. At the end of the eighties of the same century, the Germans used this chemical reaction at the first aluminum plant. In the second half of the 18th century, about 20 tons of pure metal were obtained by chemical methods. It was very expensive aluminum.
The production of aluminum using electrolysis originated in 1886, when almost identical patent applications were simultaneously filed by the founders of this method, the American scientist Hall and the Frenchman Héroux. They proposed dissolving alumina in molten cryolite and then producing aluminum by electrolysis.
This is where the aluminum industry began, which over more than a century of history has become one of the largest branches of metallurgy.
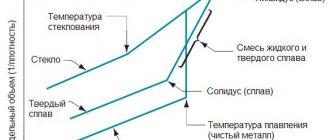
Aluminum refining process in aluminum production
Refining electrolysis with the decomposition of aqueous salt solutions is not possible for aluminum. Since the degree of purification of industrial aluminum, which is obtained by electrolysis of cryolite-alumina melt, will be insufficient for some purposes, even purer aluminum is obtained from waste metal and industrial aluminum thanks to refining. The most common refining method is three-layer electrolysis. Aluminum is used in the manufacture of explosives (alumotol, ammonal). A variety of aluminum compounds are widely used. The production and consumption of aluminum is constantly growing, greatly outpacing the growth rate of the production of copper, steel, zinc, and lead. Text, Yan Volkhovsky, promplace.ru Photo from drugoi.livejournal.com
Main stages of production technology
In general terms, aluminum production technology has not changed since its inception.
The process consists of three stages. The first of the aluminum ores, be it bauxite or nepheline, produces alumina - aluminum oxide Al2O3.
Then industrial aluminum is separated from the oxide with a purity of 99.5%, which is not enough for some purposes.
Therefore, aluminum is refined at the last stage. Aluminum production ends with its purification to 99.99%.
Famous manufacturers
The largest aluminum producer is, which produces more than 4 million tons of aluminum per year. The list of largest aluminum producers in Russia also includes:
- , specializing in working with aluminum alloys;
- JSC "BAZ", operating in the production and extraction of alumina and aluminum hydroxide;
- VgAZ, a plant for the production of primary aluminum.
Aluminum production is a complex process that requires the necessary equipment, knowledge of technology, compliance with special conditions and labor costs. But the manufacture of various aluminum products is a popular business.
This video will tell you about the prospects for aluminum production:
Alumina production
There are three ways to obtain aluminum oxide from ores:
- acidic;
— electrolytic;
- alkaline.
The last method is the most common, developed back in the 18th century, but since then repeatedly refined and significantly improved, and is used for processing high-grade bauxite. About 85% of aluminas are obtained this way.
The essence of the alkaline method is that aluminum solutions decompose at high speed when aluminum hydroxide is introduced into them. The solution remaining after the reaction is evaporated at a high temperature of about 170 ° C and again used to dissolve the alumina;
First, bauxite is crushed and ground in mills with caustic alkali and lime, then in autoclaves at temperatures up to 250 ° C, its chemical decomposition occurs and sodium aluminate is formed, which is diluted with an alkaline solution at a lower temperature - only 100 ° C. The aluminate solution is washed in special thickeners, separated from sludge. Then it decomposes. The solution is pumped through filters into containers with mixers for constant mixing of the composition, to which solid aluminum hydroxide is added for seeding.
In hydrocyclones and vacuum filters, aluminum hydroxide is released, part of which is returned as a seed material, and part is used for calcination. The filtrate remaining after separation of the hydroxide is also returned to the circulation for leaching the next batch of bauxite.
The process of calcination (dehydration) of hydroxide in rotary kilns occurs at temperatures up to 1300° C.
To produce two tons of aluminum oxide, 8.4 kWh of electricity is consumed.
A strong chemical compound with a melting point of 2050° C is not yet aluminum. Aluminum production is ahead.
Necessary equipment
In order to mine alumina in natural conditions and then extract aluminum from it, you will need a fairly large amount of equipment:
- Machines for distributing alumina;
- Cathode busbar;
- Dry gas cleaning installation;
- Electrolyzer;
- Cranes for installation, linear and technical purposes;
- Equipment necessary for foundry and anode assembly shops.
To produce aluminum, you need not only a large amount of equipment, but also a fairly large area, as well as a powerful electrical network.
The fact is that the electrolysis process takes place in special baths at a temperature of 9600C and a current of about 250,000 A.
To organize such a production process, a huge amount of electricity will be required, which is why large producers of this metal try to locate their workshops in close proximity to hydroelectric power plants that supply cheaper energy. The following is a discussion of raw materials for aluminum production.
The video below talks about aluminum production:
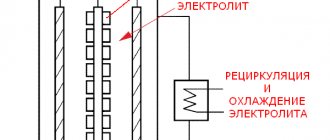
Electrolysis of aluminum oxide
The main equipment for electrolysis is a special bath lined with carbon blocks. An electric current is supplied to it. Carbon anodes are immersed in the bath, burning when pure oxygen is released from the oxide and forming carbon oxide and carbon dioxide. Baths, or electrolyzers, as experts call them, are connected in an electrical circuit in series, forming a series. The current strength is 150 thousand amperes.
Anodes can be of two types: fired from large coal blocks, the mass of which can be more than a ton, and self-burning, consisting of coal briquettes in an aluminum shell, which are sintered during the electrolysis process under high temperatures.
The operating voltage on the bath is usually about 5 volts. It takes into account both the voltage required for the decomposition of the oxide and the inevitable losses in the branched network.
From the aluminum oxide dissolved in the cryolite-based melt, the liquid metal, which is heavier than the electrolyte salts, settles on the carbon base of the bath. It is pumped out periodically.
The aluminum production process requires a lot of electricity. To obtain one ton of aluminum from alumina, you need to consume about 13.5 thousand kWh of DC electricity. Therefore, another condition for the creation of large production centers is a powerful power plant operating nearby.
Aluminum refining
The most famous method is three-layer electrolysis. It also takes place in electrolysis baths with coal beds lined with magnesite. The anode in the process is the molten metal itself, which is purified. It is located in the bottom layer on the conductive hearth. Pure aluminum, which is dissolved from the electrolyte in the anode layer, rises up and serves as the cathode. Current is supplied to it using a graphite electrode.
The electrolyte in the intermediate layer is aluminum fluorides, either pure or with the addition of sodium and barium chloride. It heats up to a temperature of 800°C.
Electricity consumption during three-layer refining is 20 kWh per kg of metal, that is, 20 thousand kWh is needed for one ton. That is why, like no other metal production, aluminum requires not just a source of electricity, but a large power plant in close proximity.
Refined aluminum contains iron, silicon, copper, zinc, titanium and magnesium in very small quantities.
After refining, aluminum is processed into commercial products. These include ingots, wire, sheets, and pigs.
The segregation products obtained as a result of refining, partly in the form of a solid sediment, are used for deoxidation, and partly are removed in the form of an alkaline solution.
Absolutely pure aluminum is obtained by subsequent zone melting of the metal in an inert gas or vacuum. Its notable characteristic is its high electrical conductivity at cryogenic temperatures.
Scope of application
As a reducing agent
Due to its chemical properties, aluminum is a strong reducing agent, as it reacts well with oxygen. This property is used for the reduction of halides and rare metals.
In ferrous metallurgy
Steel production uses aluminum and its alloys as deoxidizers, allowing not only to get rid of oxygen, but also to eliminate the possible porosity of finished products under the influence of carbon monoxide bubbles. Also in this industry it is used as alloying additives and modifiers in the form of granules, powder and powder.
Aluminum alloys
There are whole series of aluminum-based alloys that are in great demand as structural materials. These are mainly compounds with magnesium, manganese, copper, alloyed in turn with magnesium, manganese, iron and silicon. Aluminum alloys have ductility, strength, manufacturability, resistance to vibration and corrosion resistance.
Aluminum as an additive to other alloys
Aluminum is also used in alloys of other metals:
- magnesium,
- aluminum bronze,
- fechral,
- become.
Jewelry
Recently, silver-white metal has again, like a century and a half ago, begun to attract the attention of jewelers who want to add some variety to the standard set of materials used. And not only as cheap jewelry, but also as the basis for precious products, as well as independent exquisite products.
Cutlery
Aluminum cutlery is currently not as popular as before, due to reasons of harm to human health and loss of appearance during use. Although a certain number of them are present in public catering. Also, some utensils, such as spoons, forks, pots, flasks, are used as army utensils and tourist equipment.
Glass making
In the glass and glass products industry, aluminum and its compounds are widely used:
- Alumina (aluminum oxide) increases strength, hardness and resistance to temperature and chemical influences.
- Aluminum salts are necessary for the production of special types of glass.
Food industry
In addition to the food additive in E173 food products, aluminum is included in antacids intended to coat the gastrointestinal tract for pain relief in a number of diseases.
Military industry
Due to its properties: lightness and flexibility, aluminum is widely used in the designs of various types of weapons: from pistols and machine guns to tanks, missiles and aircraft. Even such exotic products for our time as crossbows, swords, rapiers, and sabers cannot do without this mineral.
In rocket technology
In addition to using aluminum as a material for making rockets, satellites and other spacecraft; powder from this metal, as well as an oxidizer based on it, are important components of solid fuel - fuel for launching shuttles and rockets.
Aluminum energy
The intermediate role of aluminum in enhancing the production of primary energy carriers or directly thermal and electrical energy manifests itself in a relatively new industry - aluminum energy. It is here, in the process of oxidation of this unique mineral, that:
- Hydrogen from water.
- Electricity - due to exposure to oxygen in the air in electrochemical generators.
Recycling of secondary raw materials
A quarter of the total demand for aluminum is met by recycling raw materials. Recycled products produce shaped castings.
Pre-sorted raw materials are melted in a threshold furnace. It retains metals that have a higher melting point than aluminum, such as nickel and iron. Various non-metallic inclusions are removed from molten aluminum by blowing with chlorine or nitrogen.
More fusible metal impurities are removed by adding magnesium, zinc or mercury and vacuuming. Magnesium is removed from the melt with chlorine.
A given casting alloy is obtained by introducing additives that are determined by the composition of molten aluminum.