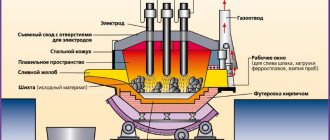
An open hearth furnace (Figure 22) has a working melting space limited from below by a hearth, from above by a roof, and from the sides by front and rear walls. The hearth has the shape of a bathtub with slopes towards the walls of the furnace. The furnace lining can be basic or acidic. If basic oxides predominate in the slag during the smelting process, the process is called the basic open-hearth process, and if the slags are acidic, the process is called acidic. The main open-hearth furnace is lined with magnesite bricks, and the acidic furnace is lined with silica bricks.
In the front wall of the furnace there are loading windows for feeding the charge, and in the rear wall there is a hole for releasing finished steel. Modern open hearth furnaces have a capacity of 200 – 900 tons of liquid steel.
The operating principle of an open-hearth furnace is shown in Figure 22 in the position of supplying fuel and air from the right side and removing combustion products through the left channels. Passing through preheated regenerator nozzles (air through an air regenerator, gas through a gas regenerator), the air and gas are heated to 1000 - 1200 °C and, in a heated state, enter the furnace through the head. When fuel burns, a torch is formed with a temperature of 1800 – 1900 °C. Having passed the head located on the opposite side of the furnace, the hot combustion products are directed to another pair of regenerator nozzles, giving off heat to them, and go into the chimney.
During this operation, the regenerator nozzles on the right side are cooled, and the nozzles on the left side are heated. At the moment when the regenerators on the right side are not able to heat the air and gas to the required temperature, the flame is automatically reversed. Cold air and gas are directed through the well-heated left regenerators, and combustion products go to the right side of the furnace, heating the cooled right regenerators. Thus, the supply and suction heads of the open-hearth furnace periodically change functions using transfer valves, and the combustion torch is formed either on the left or on the right, maintaining maximum heat recovery and avoiding overheating of the regenerator nozzles.
Gases from the regenerator enter first into the slag, and only then through a vertical channel into the furnace head. Slag traps serve to capture melting dust and slag particles carried away by combustion products from the working space, protecting the regenerator nozzles from clogging. The cross-section of the slag is larger than the cross-section of the vertical channels. Therefore, when flue gases enter the slag chambers, their speed sharply decreases and the direction of movement changes. This leads to the fact that a significant part of the smelting dust settles in the slag. When heating the gas and air entering the furnace, a high flame temperature is ensured (1800 - 1900 °C). The torch heats the working space of the furnace and promotes the oxidation of charge impurities. The higher the temperature of the gas and air entering the furnace, the higher the temperature of the torch and the better the furnace operates. However, it is possible to achieve a sufficiently high flame temperature without preheating the gas and air by enriching the air with oxygen (up to the complete replacement of air with oxygen). This leads to a decrease in the amount of combustion products and heat removal by them and, accordingly, to an increase in temperature. In this case, regenerators are unnecessary. Flue gases leave the working space of the furnace at a temperature of 1650 – 1750 °C. Entering the regenerators, the gases heat the nozzle to 1200 - 1250 °C and are removed into the chimney.
By design, open hearth furnaces are divided into:
- stationary;
- swinging.
Stationary ovens are most widespread.
Oscillating furnaces are mainly common in foundries of machine-building plants, when it is necessary to produce metal in separate portions or download large amounts of slag.
Depending on the composition of the charge used in smelting, there are different types of open-hearth process:
- scrap-ore process , in which the charge consists of liquid cast iron (55 - 75%), scrap and iron ore. The process is used in metallurgical plants that have blast furnaces;
- scrap process , in which the charge consists of steel scrap and pig iron (25 - 45%). The process is used in factories that do not have blast furnaces, but are located in industrial centers where there is a lot of scrap metal.
Scrap-ore process of steel smelting in the main open-hearth furnace.
A special feature of the main open-hearth process is that it makes it possible to produce steel with a low content of harmful impurities (phosphorus, sulfur) from ordinary charge materials.
Smelting begins with loading the solid component of the charge (iron ore, limestone, scrap) using a charging machine. After loading the solid part of the charge and heating it, liquid cast iron is poured in, which interacts with iron ore and scrap. From this moment, the period of melting of the charge begins, as a result of which, due to the oxides of ore and scrap, the impurities of cast iron (silicon, phosphorus, manganese and partially carbon) are intensively oxidized.
Silicon is oxidized and goes into slag almost completely during the melting period under the influence of an oxidizing atmosphere, as well as oxygen introduced with iron ore.
Phosphorus is oxidized simultaneously with silicon and manganese when the temperature of the metal is not yet high.
Oxides of silicon (SiO2), phosphorus (P2O5), manganese (MnO), calcium (CaO) form iron-carbon slag, which promotes the removal of phosphorus. When processing ordinary cast iron, to reduce the phosphorus content in the metal, a single download of the slag is carried out. If phosphorous cast iron is processed, then the downloading is carried out multiple times.
After melting the charge, oxidizing a significant part of the impurities and heating the metal, the boiling period of the bath begins. Iron ore is loaded into the furnace or the bath is purged with oxygen. The carbon in the metal is intensively oxidized, forming carbon monoxide (CO), which is released in the form of gas bubbles and causing the open-hearth bath to boil. This process plays a very important role, since the equalization of the composition and temperature of the metal in an open-hearth furnace is carried out by boiling the bath. During boiling, gases are removed from the metal, non-metallic inclusions float up and are absorbed by the slag, the interface between the slag and the metal increases, which helps to accelerate the process of removing harmful impurities (phosphorus, sulfur).
Due to the high oxidation of slag, removing sulfur from metal is less effective than phosphorus. To remove sulfur, new slag is added by loading lime with the addition of bauxite or fluorspar to reduce the viscosity of the slag. The CaO content in the slag increases, and FeO decreases, creating conditions for removing sulfur from the metal. To obtain steel with a low sulfur content, metal is processed using out-of-furnace methods in a ladle.
During the boiling period of the bath, carbon is intensively oxidized. Therefore, when preparing a charge for smelting, it is necessary to ensure that the carbon content in the bath at the time of melting is 0.5 - 0.6% higher than required in the finished steel. The boiling process is considered completed when the carbon content in the metal corresponds to the specified value and the phosphorus content is minimal. After this, the steel is deoxidized and, after taking control samples, it is released into a steel-pouring ladle through an opening in the rear wall of the furnace.
Steel production in open hearth furnaces
4>
Martin process (1864-1865, France). Until the seventies it was the main method of steel production. The method is characterized by relatively low productivity and the possibility of using secondary metal – steel scrap. The capacity of the furnace is 200…900 tons. The method makes it possible to produce high-quality steel.
The open hearth furnace (Fig. 2.2.) according to its design and principle of operation is a flame reverberatory regenerative furnace. Gaseous gas is burned in the smelting space
fuel or fuel oil. The high temperature for obtaining steel in a molten state is provided by heat recovery from furnace gases.
A modern open-hearth furnace is a horizontally elongated chamber made of refractory brick. The working melting space is limited from below by the hearth 12, from above by the arch 11 ,
and on the sides there are 5 front and 10 rear walls. The hearth has the shape of a bathtub with slopes towards the walls of the furnace. In the front wall there are loading windows 4 for supplying charge and flux, and in the rear wall there is a hole 9 for releasing finished steel.
A characteristic of the working space is the area of the furnace bottom, which is calculated at the level of the thresholds of the loading windows. At both ends of the melting space there are furnace heads 2, which serve to mix fuel with air and supply this mixture into the melting space. Natural gas and fuel oil are used as fuel.
To heat air and gas when operating on low-calorie gas, the furnace has two regenerators 1.
Regenerator - a chamber in which a nozzle is placed - a refractory brick laid out in a cage, designed to heat air and gases.
The gases leaving the furnace have a temperature of 1500...1600 0C. Entering the regenerator, the gases heat the nozzle to a temperature of 1250 0C. Air is supplied through one of the regenerators, which, passing through the nozzle, is heated to 1200 0C and enters the furnace head, where it is mixed with fuel; at the outlet of the head, a torch 7 is formed, directed at the charge 6.
The exhaust gases pass through the opposite head (left), cleaning devices (slag tanks), which serve to separate slag and dust particles from the gas and are sent to the second regenerator.
Cooled gases leave the furnace through chimney 8. After cooling, the nozzles of the right regenerator switch the valves, and the flow of gases in the furnace changes direction.
The temperature of the flame reaches 1800 0C. The torch heats the furnace working space and the charge. The torch promotes the oxidation of charge impurities during smelting.
Fig.2.2. Scheme of an open hearth furnace
The duration of melting is 3...6 hours, for large furnaces - up to 12 hours. The finished melt is released through a hole located in the rear wall at the lower level of the hearth. The hole is tightly plugged with low-caking refractory materials, which are knocked out when the melt is released. The furnaces operate continuously until they are stopped for major repairs - 400...600 heats.
Depending on the composition of the charge used in smelting, there are different types of open-hearth process:
– scrap process, in which the charge consists of steel scrap (scrap) and 25...45% pig iron, the process is used in factories where there are no blast furnaces, but a lot of scrap metal.
– scrap-ore process, in which the charge consists of liquid cast iron (55...75%), scrap and iron ore, the process is used in metallurgical plants with blast furnaces.
The furnace lining can be basic or acidic. If, during the steel melting process, basic oxides predominate in the slag, then the process is called basic
open-hearth process, and if acidic
- sour
.
The largest amount of steel is produced by the scrap ore process in open hearth furnaces with a main lining.
Iron ore and limestone are loaded into the furnace, and after heating, scrap is fed. After heating the scrap, liquid cast iron is poured into the furnace. During the melting period, due to ore oxides and scrap, cast iron impurities are intensively oxidized: silicon, phosphorus, manganese and, partially, carbon. The oxides form a slag with a high content of iron and manganese oxides (iron slag). After this, a period of “boiling” of the bath is carried out: iron ore is loaded into the furnace and the bath is purged with oxygen supplied through pipes 3. At this time, the supply of fuel and air to the furnace is turned off and the slag is removed.
To remove sulfur, new slag is created by applying lime with the addition of bauxite to the metal surface to reduce the viscosity of the slag. The content in the slag increases and decreases.
During the “boiling” period, carbon is intensively oxidized, so the charge must contain excess carbon. At this stage, the metal is brought to a given chemical composition, gases and non-metallic inclusions are removed from it.
Then the metal is deoxidized in two stages. First, deoxidation occurs by oxidizing the carbon of the metal, with the simultaneous supply of deoxidizing agents - ferromanganese, ferrosilicon, aluminum - to the bath. The final deoxidation with aluminum and ferrosilicon is carried out in a ladle when the steel is released from the furnace. After taking control samples, the steel is released into the ladle.
In the main open-hearth furnaces, carbon structural, low- and medium-alloy steels (manganese, chromium) are smelted, in addition to high-alloy steels and alloys, which are produced in electric melting furnaces.
High-quality steels are smelted in acidic open-hearth furnaces. A mixture with low sulfur and phosphorus content is used.
Steels contain less hydrogen and oxygen and non-metallic inclusions. Consequently, acid steel has higher mechanical properties, especially impact strength and ductility, and is used for particularly critical parts: crankshafts of large engines, rotors of powerful turbines, ball bearings.
The main technical and economic indicators of steel production in open hearth furnaces are:
· furnace productivity – steel removal from 1 m2 of hearth area per day (t/m2 per day), on average 10 t/m2; R
· fuel consumption per 1 ton of steel produced is on average 80 kg/t.
As furnaces become larger, their economic efficiency increases.
Steel production in oxygen converters.
The oxygen-converter process is the smelting of steel from liquid cast iron in a converter with a main lining and blowing oxygen through a water-cooled lance.
The first experiments in 1933-1934 - Mozgovoy.
On an industrial scale - in 1952-1953 at factories in Linz and Donawitz (Austria) - it was called the LD process. Currently, the method is the main one in the mass production of steel.
An oxygen converter is a pear-shaped vessel made of steel sheet, lined with base brick.
Converter capacity is 130...350 tons of liquid cast iron. During operation, the converter can be rotated 360° to load scrap, pour cast iron, drain steel and slag.
The charge materials of the oxygen-converter process are liquid pig iron, steel scrap (no more than 30%), lime for slag removal, iron ore, as well as bauxite and fluorspar for slag liquefaction.
The sequence of technological operations when melting steel in oxygen converters is presented in Fig. 2.3.
Fig.2.3. The sequence of technological operations when melting steel in oxygen converters
After the next steel melting, the outlet hole is sealed with a refractory mass and the lining is inspected and repaired.
Before melting, the converter is tilted and scrap rice is loaded using charging machines. (2.3.a), cast iron is poured at a temperature of 1250...1400 0C (Fig. 2.3.b).
After this, the converter is turned to the working position (Fig. 2.3.c), a cooled lance is inserted inside and oxygen is supplied through it at a pressure of 0.9...1.4 MPa. Simultaneously with the start of blowing, lime, bauxite, and iron ore are loaded. Oxygen penetrates the metal, causing it to circulate in the converter and mix with the slag. A temperature of 2400 0C develops under the tuyere. In the zone of contact of the oxygen jet with the metal, iron is oxidized. Iron oxide dissolves in the slag and metal, enriching the metal with oxygen. Dissolved oxygen oxidizes silicon, manganese, and carbon in the metal, and their content decreases. The metal is heated by the heat released during oxidation.
Phosphorus is removed at the beginning of purging the bath with oxygen, when its temperature is low (the phosphorus content in cast iron should not exceed 0.15%). If the phosphorus content is high, to remove it, it is necessary to drain the slag and introduce a new one, which reduces the productivity of the converter.
Sulfur is removed throughout the entire melting process (the sulfur content in cast iron should be up to 0.07%).
The oxygen supply is stopped when the carbon content in the metal corresponds to the specified value. After this, the converter is turned and the steel is released into a ladle (Fig. 2.3.d), where it is deoxidized using the precipitation method with ferromanganese, ferrosilicon and aluminum, then the slag is drained (Fig. 2.3.e).
In oxygen converters, steels with different carbon contents, boiling and calm, as well as low-alloy steels are smelted. Alloying elements in molten form are introduced into the ladle before steel is released into it.
Melting in converters with a capacity of 130...300 tons ends in 25...30 minutes.
Steel production in electric furnaces
Electric melting furnaces have advantages compared to other melting units:
a) it is easy to regulate the thermal process by changing current parameters;
b) it is possible to obtain a high temperature of the metal,
c) the ability to create an oxidizing, reducing, neutral atmosphere and vacuum, which allows the metal to be deoxidized with the formation of a minimum amount of non-metallic inclusions.
Electric furnaces are used for smelting structural, high-alloy, tool, and special alloys and steels.
There are arc and induction electric furnaces.
Arc melting furnace.
The arc furnace diagram is shown in Fig. 3.1.
Fig.3.1. Arc melting furnace diagram
The arc furnace is powered by three-phase alternating current. Has three cylindrical electrodes 9
from a graphitized mass, fixed in electrode holders
8
, to which electric current is supplied via cables
7
.
An electric arc occurs
between the electrode and the metal charge 3 The furnace body has the shape of a cylinder. Outside it is enclosed in a durable steel casing 4
, inside it is lined with basic or acid brick
1
.
The melting space is limited by walls 5
, hearth
12
and roof
6.
The removable roof
6
has holes for electrodes.
In the wall of the housing there is a working window 10
(for draining slag, loading ferroalloys, taking samples), closed during melting with a shutter.
The finished steel is discharged through a drain hole with a drain chute 2
.
The furnace rests on sectors and has a drive 11
for tilting towards the working window or chute. The furnace is loaded with the roof removed.
The capacity of the furnaces is 0.5…400 tons. In metallurgical shops, electric furnaces with a basic lining are used, and in foundries - with an acid lining.
In the main arc furnace, two types of smelting are carried out:
a) on a charge from alloyed waste (by remelting method),
b) on a carbonaceous charge (with oxidation of impurities).
Melting on a charge made from alloyed waste
conduct without oxidation of impurities. After melting the charge, sulfur is removed from the metal, introducing the main slag, if necessary, it is carburized and the metal is brought to the specified chemical composition. Diffusion deoxidation is carried out by feeding crushed ferrosilicon, aluminum, and ground coke onto the slag. This is how alloy steels are smelted from waste from machine-building plants.
Melting on a carbon charge
used for the production of structural steels. The furnace is loaded with the following mixture: steel scrap, pig iron, scrap electrodes or coke for carburizing metals and lime. The electrodes are lowered and the current is turned on. The charge melts under the action of the electrodes, and the metal accumulates in the bottom of the furnace. During the melting of the charge, air oxygen, charge oxides and scale oxidize iron, silicon, phosphorus, manganese, and partially carbon. Calcium oxide from lime and iron oxide form the main ferrous slag, which helps remove phosphorus from the metal. After heating to 1500...1540 0C, ore and lime are loaded, a period of “boiling” of the metal is carried out, and further oxidation of carbon occurs. After boiling stops, the slag is removed. Then they begin to remove sulfur and deoxidize the metal of a given chemical composition. Deoxidation is carried out by precipitation and diffusion method. To determine the chemical composition of the metal, samples are taken and, if necessary, ferroalloys are introduced into the furnace to obtain the desired chemical composition. Then final deoxidation is performed with aluminum and silico-calcium, and the steel is released into the ladle.
When smelting alloy steels in arc furnaces, alloying elements in the form of ferroalloys are introduced into the steel.
High-quality carbon steels - structural, tool, heat-resistant and heat-resistant - are smelted in arc furnaces.
Induction Crucible Melting Furnaces
They smelt the highest quality corrosion-resistant, heat-resistant and other steels and alloys.
Capacity from tens of kilograms to 30 tons.
The diagram of an induction crucible furnace is shown in Fig. 3.2.
Rice. 3.2. Diagram of an induction crucible furnace
The furnace consists of a water-cooled inductor 3
, inside of which there is a crucible
4
(basic or acidic refractory materials) with a metal charge, a single-phase alternating current of high frequency (500...2000 Hz) passes through the inductor from a high-frequency generator.
When passing current through an inductor in metal 1
located in the crucible, powerful eddy currents are induced, which ensures heating and melting of the metal. To reduce heat loss, the stove has a removable roof
2
.
The crucible is made from acidic (quartzite) or basic (magnesite powder) refractories. To release the melt, the furnace is tilted towards the drain chute.
Under the influence of the electromagnetic field of the inductor during melting, intensive circulation of liquid metal occurs, which helps to accelerate chemical reactions, obtain a metal with a homogeneous chemical composition, quickly float up non-metallic inclusions, and equalize the temperature.
In induction furnaces, steel and alloys are smelted from alloyed waste using the remelting method.
, or from pure charge iron and scrap with the addition of ferroalloys
by fusion
.
After melting the charge, a slag mixture is loaded onto the surface of the metal to reduce heat losses of the metal and reduce the loss of alloying elements, protecting it from saturation with gases.
When smelting in acid furnaces, after melting and removing the smelting slag, slag is removed from broken glass. For final deoxidation, ferrosilicon, ferromanganese and aluminum are introduced into the ladle before releasing the metal.
In the main furnaces, deoxidation is carried out with a mixture of powdered lime, coke, ferrosilicon, ferromanganese and aluminum.
In the main furnaces, high-quality alloy steels with a high content of manganese, titanium, nickel, and aluminum are smelted, and in acid-lined furnaces, structural steels alloyed with other elements are smelted.
In furnaces it is possible to produce steels with low carbon content and carbon-free alloys, since there is no carburizing medium.
In vacuum induction melting, the inductor, crucible, batch dispenser and molds are placed in vacuum chambers. High-quality alloys with a low content of gases, non-metallic inclusions and alloys alloyed with any elements are obtained.
Steel casting
From the melting furnaces, steel is released into a ladle, which is carried by an overhead crane to the steel casting site. From the ladle, the steel is poured into the molds or molds of a continuous casting machine. In molds or crystallizers, steel hardens and ingots are obtained, which are rolled and forged.
Molds
– cast iron molds for the production of ingots.
Molds are made with square, rectangular, round and multifaceted cross sections.
Ingots with a square cross-section are converted into long products: I-beams, channels, angles. Rectangular ingots - into sheets. Round ingots are used to make pipes and wheels. Ingots with a multifaceted cross-section are used for the manufacture of forgings.
Calm and boiling carbon steels are poured into ingots weighing up to 25 tons, alloy and high-quality steels into ingots weighing 0.5...7 tons, and some types of high-alloy steels into ingots weighing up to several kilograms.
Steel is poured into molds from above (Fig. 3.3.a), from below (by siphon) (Fig. 3.3.b) and on continuous casting machines (Fig. 3.4).
Fig.3.3. Pouring steel into molds
a – from above; b – from below (siphon)
Steel is poured into the molds from above directly from the ladle. 1
. This eliminates the consumption of metal on the gates and simplifies the preparation of equipment for casting. The disadvantages include the poorer quality of the surface of the ingots, due to the presence of oxide films from metal splashes that harden on the walls of the mold.
Used for casting carbon steels.
For siphon casting
Several molds (4…60) are filled simultaneously.
The molds are installed on a pallet 6
, in the center of which there is a center sprue
3,
lined with refractory tubes
4
, connected by channels
7
to the molds.
Liquid steel 2
from ladle
1
enters the center sprue and smoothly, without splashing, fills the mold
5
.
The surface of the ingot is clean, and a large mass of metal can be poured simultaneously into several molds.
Used for alloy and high-quality steels.
Continuous casting
steel is that liquid steel from ladle
1
through an intermediate casting device
2
is continuously fed into a water-cooled mold without a bottom - crystallizer
3
, from the bottom of which a solidifying ingot
5
.
Before pouring the metal into the crystallizer, a seed is introduced - a steel rod with a replaceable head having a dovetail-shaped groove, which at the beginning of pouring serves as the bottom of the crystallizer. Due to intense cooling, the liquid metal at the walls of the crystallizer and on the seed solidifies, and a crust forms, connecting the metal with the seed. The seed moves downwards using traction rollers 6
, gradually pulling the solidifying ingot out of the crystallizer.
After passing through the traction rollers 6
, the seed is separated.
The pulling speed is on average 1 m/min. Final hardening in the core occurs as a result of secondary cooling with water from the spray 4
.
Then the hardened ingot enters the cutting zone, where it is cut with a gas cutter 7
into pieces of a given length. The ingots have a dense structure and a fine-grained structure; there are no shrinkage cavities.
Fig.3.4. Scheme of continuous steel casting
Ways to improve the quality of steel
The quality of metal can be improved by reducing harmful impurities, gases, and non-metallic inclusions in it. To improve the quality of metal, they use: treatment with synthetic slag, vacuum degassing of metal, electroslag remelting (ESR), vacuum arc remelting (VAR), metal remelting in electron arc and plasma furnaces, etc.
Vacuum degassing
carried out to reduce the content of gases and non-metallic inclusions in the metal.
Vacuuming
steel is carried out in a ladle, when pouring from ladle to ladle, when pouring into a mold.
To vacuum the ladle, the ladle with liquid steel is placed in a chamber closed with a sealed lid. Vacuum pumps create a vacuum to a residual pressure of 0.267...0.667 kPa. When the pressure decreases, hydrogen and nitrogen are released from the liquid steel. The floating gas bubbles capture non-metallic inclusions, as a result of which their content in the steel decreases. The strength and ductility of steel improves.
Electroslag remelting (ESR))
used for smelting high-quality steels for bearings and heat-resistant steels.
The diagram of electroslag remelting is shown in Fig. 3.5.
Metal melted in an arc furnace and rolled into a rod is subjected to remelting. The heat source is a slag bath heated by electric current. Electric current is supplied to the electrode being melted 1
immersed in a slag bath
2
, and to a tray
9
installed in a water-cooled crystallizer
7
, which contains a seed
8
.
The released heat heats bath 2
to a temperature above 1700? C and causes melting of the end of the electrode.
Drops of liquid metal 3
pass through the slag and form a metal bath
4
. The transfer of metal droplets through the main slag helps remove sulfur, non-metallic inclusions and gases from the metal. The metal bath is replenished by melting the electrode, and under the influence of the crystallizer it is gradually formed into ingot 6. The oxygen content decreases by 1.5...2 times, sulfur by 2...3 times. The ingot is distinguished by density, uniformity, good surface quality, and high mechanical and performance properties. Ingots are produced in round, square and rectangular sections, weighing up to 110 tons.
Fig.3.5. Electroslag remelting circuit
Vacuum arc remelting (VAR)
used to remove gases and non-metallic inclusions from metal.
The process is carried out in vacuum arc furnaces with a consumable electrode. The cathode is produced by mechanical processing of an ingot smelted in electric furnaces or ESR installations.
The diagram of vacuum-arc remelting is shown in Fig. 3.6.
Consumable electrode 3
fixed on a water-cooled rod
2
and placed in the furnace body
1
and then in a copper water-cooled mold
6
.
Air is pumped out of the furnace body to a residual pressure of 0.00133 kPa. When voltage is applied between the consumable electrode 3
(cathode) and the seed
8
(anode), an arc occurs.
The generated heat melts the end of the electrode. Drops of liquid metal 4
, passing through the arc discharge zone, are degassed, fill the mold and solidify, forming an ingot
7
.
The arc burns between the electrode and liquid metal 5
in the upper part of the ingot throughout the entire melting process. Cooling the ingot and heating the liquid metal creates conditions for directed solidification of the ingot. Consequently, non-metallic inclusions are concentrated in the upper part of the ingot, and the shrinkage cavity is small.
Fig.3.6. Scheme of vacuum-arc remelting
The ingot is characterized by high uniformity of chemical composition and improved mechanical properties. They produce parts for turbines, engines, and aircraft structures. The weight of the ingots reaches 50 tons.
Production of non-ferrous metals
Non-ferrous metals are used in various industries: copper, nickel, zinc, lead, aluminum, titanium, tin, as well as other metals and their alloys. In many cases, the properties of alloys are superior to those of pure metals.
Alloys of copper and zinc form a large group of different brasses that have higher strength than pure copper.
Alloys of copper with other metals (except zinc) are called bronzes.
Depending on their specific gravity, non-ferrous metals are classified as heavy non-ferrous metals (lead, nickel, copper, etc.) and light non-ferrous metals (aluminum, magnesium, titanium).
Copper production
Copper in nature is found in the form of sulfur compounds, oxides, bicarbonates, carbon dioxide compounds in sulfide ores and native copper metal.
The most common ores are copper pyrite and copper luster, containing 1...2% copper.
90% of primary copper is obtained by pyrometallurgical method, 10% by hydrometallurgical method.
Hydrometallurgical method–
obtaining copper by leaching it with a weak solution of sulfuric acid and subsequent separation of copper metal from the solution.
Obtaining copper by pyrometallurgical method
consists of enrichment, roasting, matte smelting, purging in a converter, and refining.
Enrichment
Copper ores are produced by flotation and oxidative roasting.
Flotation method
is based on the use of different wettabilities of copper-containing particles and waste rock. Allows you to obtain copper concentrate containing 10...35% copper.
Copper ores and concentrates containing large amounts of sulfur are subjected to oxidative roasting
. In the process of heating the concentrate or ore to 700...800 0C in the presence of atmospheric oxygen, sulfides are oxidized and the sulfur content is reduced by almost half the original value. Only poor concentrates (with a copper content of 8...25%) are fired, and rich (25...35% copper) concentrates are melted without firing.
After roasting, the ore and copper concentrate are smelted into matte
, which is an alloy containing copper and iron sulfides. The matte contains 20...50% copper, 20...40% iron, 22...25% sulfur, about 8% oxygen and admixtures of nickel, zinc, lead, gold, and silver. Most often, smelting is carried out in fiery reverberatory furnaces. The temperature in the melting zone is 1450 0C.
The resulting copper matte, in order to oxidize sulfides and iron, is blown with compressed air in horizontal converters with side blast. The resulting oxides are converted into slag, and sulfur into. Heat in the converter is released due to chemical reactions without fuel supply. The temperature in the converter is 1200…1300?C. Thus, blister copper
, containing 98.4...99.4% copper, 0.01...0.04% iron, 0.02...0.1% sulfur and a small amount of nickel, tin, antimony, silver, gold. This copper is poured into a ladle and poured into steel molds or a casting machine.
Blister copper is refined to remove harmful impurities, and fire
and then
electrolytic refining
.
The essence of fire refining
blister copper consists of oxidizing impurities that have a greater affinity for oxygen than copper, removing them with gases and converting them into slag. After fire refining, copper of 99...99.5% purity is obtained. It is poured into molds and ingots are obtained for further smelting of alloys (bronze and brass) or ingots for electrolytic refining.
Electrolytic refining
carried out to obtain copper free of impurities (99.95%).
Electrolysis is carried out in baths where the anode is made of fire-refined copper, and the cathode is made of thin sheets of pure copper. The electrolyte is an aqueous solution (10...16%) and (10...16%).
When a direct current is passed, the anode dissolves, the copper goes into solution, and copper ions are discharged at the cathodes, depositing a layer of pure copper on them.
Impurities are deposited at the bottom of the bath in the form of slag, which is processed to extract metals.
The cathodes are unloaded after 5...12 days, when their mass reaches 60...90 kg. They are thoroughly washed and then melted in electric furnaces.
Copper by purity is divided into grades: M0 (99.95% Cu), M1 (99.9%), M2 (99.7%), M3 (99.5%), M4 (99%).
Titanium production
Titanium is a metal, an element of group 4 of D.I. Mendeleev’s Periodic Table. Density 4.5 g/cm3, melting point 1665°C, boiling point -3672°C, tensile strength of pure titanium 20 mPa, commercial titanium 300-400 mPa, structural alloys -120-140 mPa. Titanium interacts with most elements, which creates difficulties in obtaining pure titanium, and on the other hand, makes it possible to obtain a large number of alloys of varying composition and properties. The combination of high strength and high corrosion resistance makes titanium one of the best structural materials (aircraft, marine vessels, chemical engineering). Despite the high cost, its use pays off with the service life of the equipment made from it.
The titanium content in the earth's crust is 0.61%. In terms of prevalence among metals, it ranks fourth after aluminum (8.13%), iron (5%) and magnesium (2.1%). There are about eighty minerals that contain titanium. The most common minerals are ilmenite (FeO.TiO2 - containing 3-59%TiO2) and rutile (90-100%TiO2). Most titanium ores are complex and, along with titanium, contain a number of other valuable minerals, and therefore the ores cannot be used without beneficiation. The most commonly used are flotation, as well as magnetic separation, washing and wet concentration. As a result of enrichment, iron-titanium concentrates are obtained with a titanium dioxide content of 42-63% and 18-35% iron, some of which is in oxide form. The processing of these concentrates involves the production of titanium dioxide, but also the production of cast iron (steel). The main raw material for the production of titanium metal is titanium slag obtained as a result of ore reduction smelting. For one ton of titanium slag, 0.25 to 0.7 tons of cast iron are obtained. The technology for the production of titanium slag and cast iron from iron-titanium concentrates includes the following main stages:
1. Preparing the charge;
2. Reducing electric smelting;
3. Cutting and processing of smelting products.
Titanium slag can be smelted from powder, briquetted and rounded charge. In the latter case, grinding of the starting materials is required. Therefore, powder or briquetted mixture is used. The concentrate and reducing agent (anthracite) from the factory warehouse of raw materials enter the trenches or bunkers of the charge department of the workshop, where
preparation of charge for electric smelting. Preparation includes the following stages:
- crushing and grinding of the reducing agent,
-dosing of charge components, mixing,
- dosage of the binder and mixing with heating of the charge,
-briquetting on a roller press.
Reductive electric smelting is carried out in electric arc ore-smelting furnaces at temperatures of 1650-1750°C. Iron reduction occurs at temperatures of 900-1050 oC. Along with the reduction of iron oxides at 1050 °C and above, titanium dioxide is reduced to lower oxides:
3(Fe TiO2)+4C=3Fe+Ti3O5+4CO
2(FeO TiO2)+C=Fe+FtO 2TiO2+CO
Iron is saturated with reducing agent carbon and sinks to the bottom of the bath, and titanium oxides (up to 90%) with a small amount of oxides Fe, Mn, Si, Ca, Mg, Al, Cr turn into slag. Cast iron is released after 7-8 hours, and slag after 3.5-4 hours.
The resulting slag is used to produce titanium tetrachloride, which is the next step before obtaining titanium metal.
Preparation of titanium tetrachloride
Chlorination is a process in which chlorine reacts with oxides of elements, the resulting chlorides have a melting and boiling point significantly lower than the melting and boiling points of the oxides of the corresponding elements. This important property of chlorides makes it possible to isolate certain useful components at lower temperatures and using simpler technological methods. During the chlorination process, the oxides of titanium-containing minerals interact with chlorine and carbon and are converted into chlorides at a temperature of 600-1200 oC. In a simplified way, the chemical reaction of chlorination of titanium dioxide can be represented as:
TiO2+2COCl2=TiCL4+2CO2
Chlorination can be carried out in various devices:
- mine furnaces;
- in devices with a liquid bath of molten alkali metal chlorides;
- in devices with a pseudo-boiling bed.
Technical titanium tetrachloride obtained during chlorination contains a significant amount of dissolved and suspended impurities. Its purification is carried out in several stages depending on the chemical composition of the impurities. Purification is carried out by filtration, freezing, in distillation columns.
Recovery of titanium tetrachloride
The reduction is carried out in a steel reactor with magnesium in an atmosphere of inert gas (argon or helium). Magnesium is loaded into the reactor in liquid or solid form immediately in the amount required for the entire process. Titanium tetrachloride is fed into the reactor continuously throughout the entire process in liquid form.
TiCl4+2Mg=Ti+2MgCl2
After reduction, a sintered block of reaction mass remains in the reactor, consisting of a titanium sponge impregnated with magnesium and magnesium chloride. The block is firmly welded to the reactor walls. The sponge is removed from the reactor by drilling on special machines, after which it is subjected to separation, additional cleaning and remelting in vacuum arc furnaces
Magnesium production
To obtain magnesium, the most widely used method is the electrolytic method, the essence of which is to obtain pure anhydrous magnesium salts, electrolysis of these salts in a molten state and refining of metallic magnesium.
The main raw materials for the production of magnesium are: carnallite, magnesite, dolomite, bischofite. The largest amount of magnesium is obtained from carnallite. First, the carnallite is enriched and dehydrated. Anhydrous carnallite is used to prepare an electrolyte.
Electrolysis is carried out in an electrolyzer lined with fireclay bricks. The anodes are graphite plates, and the cathodes are steel plates. The electrolyzer is filled with molten electrolyte of composition 10%, 45%, 30%, 15%, with small additives etc. This composition of the electrolyte is necessary to lower its melting point (720 0C). To electrolytically decompose magnesium chloride, current is passed through the electrolyte. As a result, chlorine ions are formed, which move towards the anode. Magnesium ions move to the cathode and, after discharge, are released on the surface, forming droplets of liquid rough magnesium. Magnesium has a lower density than the electrolyte, so it floats to the surface, from where it is periodically removed with a vacuum ladle.
Raw magnesium contains 5% impurities, so it is refined by remelting with fluxes. To do this, rough magnesium and flux, consisting of , are heated in a furnace to a temperature of 700...750 0C and mixed. In this case, non-metallic impurities turn into slag. Then the furnace is cooled to a temperature of 670 0C and magnesium is poured into molds into pigs.
Procurement production.
The need to save material resources places high demands on the rational selection of workpieces, the level of their manufacturability, and, to a large extent, determining
4>
Date added: 2016-09-26; views: 5011; ORDER A WORK WRITING
Find out more:
Acid open hearth process.
Currently, the acid open-hearth process has limited use due to the high requirements for the purity of the charge. In an acid furnace, the process is carried out with acidic slag, so it is impossible to remove sulfur and phosphorus from the metal. To conduct the acid process, high-quality charcoal or coke cast iron is used, in which the content of harmful impurities does not exceed 0.025%.
Scrap metal coming from other enterprises is melted down in the main furnaces to obtain a charge billet, which is loaded instead of scrap and intermediate products when the metal is poured into the acid furnace in liquid form. The liquid intermediate is released from the main furnace into a ladle and then poured into the acid furnace. This process is called a duplex process, since it involves two units - the main and acidic open-hearth furnaces.
The fuel in the acid process must contain a minimum amount of sulfur. Steels smelted in acid open-hearth furnaces contain less non-metallic inclusions, hydrogen and oxygen than those smelted in the main furnace. Therefore, acid steel has higher mechanical properties, especially impact strength and ductility, and is used for particularly critical parts (crankshafts of large engines, artillery pieces, rotors of powerful turbines).
Steel production in two-tub steelmaking units.
Double-bath steelmaking units have two baths connected by a channel for transition from one bath to another (Figure 23). The operating principle of a two-bath oven is as follows. When the metal is purged with oxygen in one bath after cast iron is poured, in another bath the metal is filled and the solid charge is heated with gases escaping from the first bath. After releasing the metal from the first bath, the charge is filled. At the same time, the second bath begins to be purged with oxygen. Fuel is supplied to the two-bath units through fuel-oxygen burners installed in the roof and ends of the furnace. If the charge contains more than 65% liquid cast iron, then the two-bath furnace can operate without fuel consumption, since the amount of physical heat and heat released during the oxidation of cast iron impurities, as well as the oxidation of CO to CO2, increases. In this case, the two-bath furnace becomes similar to an oxygen converter.
The quality of the metal produced in two-bath units does not differ from the quality of open-hearth or oxygen-converter steel. The technical and economic indicators of the process in two-bath steelmaking units are characterized by:
- high performance;
- low specific consumption of fuel and refractories.
The main disadvantages of the process that limit its widespread use include:
- higher consumption of liquid iron compared to the open-hearth scrap-ore process;
- higher iron waste;
- limited range of smelted metal.
Technology
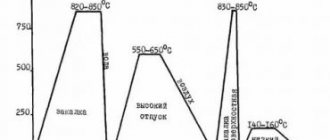
The periods of the steel production process in an open-hearth furnace last from five to eight hours (with high-speed steelmaking - up to 4.5-5.5 hours) and consist of the following stages:
- Melting
. Melting begins even before the furnace is loaded. They try to carry out melting at the maximum temperature in order to prevent gases from dissolving in the metal and to prevent excessive oxidation. During this period, silicon, manganese, iron, phosphorus are intensively oxidized, and a large amount of ferric oxide F e O {displaystyle mathrm {FeO} } is formed. - Oxidation
. Carbon oxidation occurs due to the previously formed F e O {displaystyle mathrm {FeO} }. Reaction formula: C + F e O = CO + F e − Q {displaystyle mathrm {C+FeO=CO+Fe-Q} } . The resulting carbon monoxide CO {displaystyle mathrm {CO} } causes the melt to boil. Within 2-3 hours, the proportion of carbon in the melt decreases and becomes below 2%. - Deoxidation
. If by the end of melting a large amount of F e O {displaystyle mathrm {FeO} } is dissolved in the steel, this gives the steel brittleness when hot - red brittleness. To remove oxygen, steel is deoxidized with ferrosilicon, ferromanganese or aluminum. Sometimes, to check, a red-hot piece of steel is forged; if it is poorly deoxidized, cracks form. - enriching the flame during the filling and melting of charge materials;
- blowing through a liquid bath during the carbon burnout period.
If necessary, after deoxidation, alloying elements are introduced: ferrotitanium, ferrochrome, high-silicon ferrosilicon, pure nickel and others.
After melting is completed, the steel is released into the ladle.
To speed up the process and increase productivity by 15-25%, oxygen is used. It is introduced during melting in two ways: