Features of the steel production process
In the production of cast iron and steel, different technologies are used, despite the fairly similar chemical composition and some physical and mechanical properties. The differences are that steel contains less harmful impurities and carbon, due to which high performance is achieved. During the smelting process, all impurities and excess carbon, which causes an increase in the fragility of the material, go into slag. Steel production technology involves forced oxidation of basic elements due to the interaction of iron with oxygen.
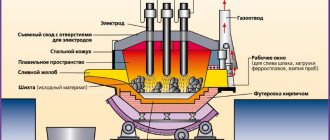
Melting steel in an electric furnace
When considering the production process of carbon and other types of steel, several main stages of the process should be highlighted:
- Rock melting. The raw materials that are used to produce metal are called charge. At this stage, during the oxidation of iron, impurities are also deoxidized. Much attention is paid to reducing the concentration of harmful impurities, which include phosphorus. To ensure the most suitable conditions for the oxidation of harmful impurities, a relatively low temperature is initially maintained. The formation of iron slag occurs by adding iron ore. After the release of harmful impurities on the surface of the alloy, they are removed, and a new portion of calcium oxide is added.
- Boiling of the resulting mass. After the preliminary stage of cleaning the composition, baths of molten metal are heated to a high temperature, and the alloy begins to boil. Due to boiling, the carbon contained in the composition begins to actively oxidize. As previously noted, cast iron differs from steel in having too high a carbon concentration, due to which the material becomes brittle and acquires other properties. This problem can be solved by injecting pure oxygen, due to which the oxidation process will occur at high speed. When boiling, bubbles of carbon monoxide are formed, to which other impurities also adhere, due to which the composition is purified. At this stage of production, sulfur, which is a harmful impurity, is removed from the composition.
- Deoxidation of the composition. On the one hand, adding oxygen to the composition ensures the removal of harmful impurities, on the other hand, it leads to a deterioration in basic performance qualities. That is why, to clean the composition from harmful impurities, diffusion deoxidation is often carried out, which is based on the introduction of a special molten metal. This material contains substances that have approximately the same effect on the molten alloy as oxygen.
In addition, depending on the characteristics of the technology used, two types of materials can be obtained:
- Calm ones who have gone through the deoxidation process to the end.
- Semi-quiet, which have a state between calm and boiling steels.
During the production of the material, pure metals and ferroalloys can be added to the composition. Due to this, alloyed compounds are obtained that have their own specific properties.
Advantages and disadvantages of DC and AC arc furnaces
- home
- >
- Library
- >
- Steel production in chipboard
The first arc steel furnaces, which appeared at the end of the 19th century, were direct current furnaces. Later, AC furnaces dominated the iron and steel industry for many decades. And despite the successes achieved, AC arc furnaces have a number of disadvantages:
- they operate with a relatively low power factor,
- are sources of powerful interference in power supply systems,
- cause severe pollution of the environment,
- have a high noise level.
To eliminate these shortcomings, since the early 80s, DC arc furnaces have become increasingly widespread.
Until 1990, the number of these furnaces was few; by the beginning of 1993, there were 46 DC arc furnaces in operation in the world, and by the end of 1998, there were more than 130 DC arc steelmaking furnaces.
In the design and operation of DC EAFs, many new developments were used, both electrical and design, as well as technological: new designs of transformers, rectifiers, tuyeres, burners, new automation and control systems, methods of injecting oxygen, carbon, slag-forming agents, heating and loading of the charge, afterburning of process gases, bottom eccentric release of melts, foaming of slags, bottom purging of the bath with gases, etc.
The duration of smelting in new large furnaces is less than 60 minutes and it is expected to increase it in the near future to 30 - 40 minutes.
The main differences between DC furnaces from different companies are the number of bottom electrodes - cathodes and the design of the current-carrying unit - anode. Multi-electrode DC furnaces have not become widespread. Almost all operating DC arc furnaces are single-electrode.
According to the design, the current-carrying units of the hearth can be mainly divided into four types (in parentheses - the developer company):
- conductive hearth, air cooling (ABB Industrie AG);
- multi-rod metal bottom electrode, air cooling (MAN GHH / Nippon Kokan);
- multi-plate metal bottom electrode, air cooling (Deutshe Voest Alpine Industrieanlagenbau);
- single-rod metal electrode, sometimes several electrodes, water cooling (Clecim).
No particular advantages have been identified for any of these hearth electrodes; Their durability, as a rule, exceeds 1200 heats.
The power line of DC arc furnaces, unlike three-phase furnaces, includes a rectifier unit and a smoothing reactor, which reduces the flicker effect. For DC power supply, thyristor and diode converters are used. I have a clear opinion about that. Which of these converters is better is currently not known. The world has mastered the production of powerful and compact rectifiers for furnaces of any capacity. If the unit power of the converters is insufficient, they are combined into blocks to obtain the necessary parameters of the power supplies. Power supplies can be assembled according to serial, parallel or parallel-series connection schemes. This increases reliability, since if one transformer fails, the melting process can be continued using the remaining one. In addition, a mode with increased voltage when connected in series and increased arc current when connected in parallel of thyristor sections can be implemented without increasing the installed power of the power supply. In the bottom of an arc furnace, you can place several electrodes isolated from the body and create high-power furnaces by connecting each power source to a specific bottom electrode.
DC furnaces have the following advantages over AC furnaces:
- lower specific consumption of electrodes by 50-60%;
- reduction in flicker level by 50%;
- the ability to supply more power;
- higher reliability of electrical equipment;
- working with long arcs;
- stirring the bath under the influence of electrodynamic forces;
- simplified maintenance and reduced labor costs;
- uniform thermal load on the furnace lining;
- noise level reduction by 15 dB;
- technology stabilization;
- better formation of wells when melting the charge;
- reduction of waste of alloying elements;
- reduction of nitrogen content in steel:
- reduction of gas emission and dust formation;
- reduction in refractory consumption;
- productivity increase.
However, despite the widely advertised benefits of DC furnaces, some companies prefer to install three-phase furnaces.
This is due to the following:
- capital costs for an AC furnace are lower;
- the total energy consumption is almost the same;
- the end consumption of electrodes and the impact on the supply network of high-impedance three-phase furnaces and DC furnaces are approaching;
- AC furnaces have greater flexibility in controlling bath temperature.
Disadvantages of DC arc furnaces:
- work on long arcs leads to increased heat losses (a direct current arc is characterized by a reduced potential gradient in the arc column, which, in order to ensure the required power is released in the arc, necessitates increasing its length to 0.8 -1.0 m. After melting the charge, this leads to to an increase in radiation fluxes on the walls and roof of the furnace and an increase in heat losses);
- in a DC arc furnace, special measures are required to prevent arc deflection due to the phenomenon of magnetic blast;
- Introducing a semiconductor source into the electrical circuit reduces the reliability of installations and increases their cost.
Similarly, the alternating current arc does not meet the electrotechnological requirements. During the initial period of melting, the arc has a short length, which increases the likelihood of operational short circuits and increases energy consumption, increasing the impact on the supply network. At the same time, the arc continuously moves, which expands the wells and reduces the severity of the problem of hearth overheating. When melting the charge, an alternating current arc is more efficient (without taking into account the consumption of electrodes).
Studies have shown that the efficiency of AC arcs, depending on the electrical and slag modes, varies within 0.55 - 0.85, the efficiency of DC arcs - within 0.40 - 0.75, which explains the higher, in some cases, specific consumption electricity in DC arc furnaces.
The performance indicators of modern DC and AC arc furnaces are similar, with the exception of the consumption of electrodes - on DC arc furnaces, the consumption of graphite electrodes is approximately two times lower than on AC arc furnaces.
In the near future, the “competition” between DC and AC arc furnaces will continue; both furnaces will be built, but DC arc furnaces will be built at a faster pace - approximately twice as many as new AC arc furnaces.
When determining the type of furnace to be built in each specific case, a comprehensive analysis of the project is carried out, which takes into account
- geographical location of the site where the furnace will be installed;
- type of metal charge used;
- availability of powerful sources of electricity;
- provision of fuel and oxygen;
- environmental requirements;
- the possibilities of refining liquid metal in out-of-furnace processing units and other factors.
All other things being equal, preference is given to technologies and units characterized by lower costs for raw materials and their transportation.
- ← Section 13.1
- Section 13.2.2.1 →
Steel production methods
There are several methods for producing steel, each with its own specific advantages and disadvantages. The chosen method determines what properties the material can be obtained with. Main methods of steel production:
- Martin's method. This technology involves the use of special furnaces that are capable of heating raw materials to a temperature of about 2000 degrees Celsius. Considering the methods for producing alloy steels, we note that this method also allows the addition of various impurities, due to which steels of unusual composition are obtained. The open hearth method is based on the use of special furnaces.
- Electric steel melting method. In order to obtain high quality material, steel is produced in electric furnaces. By using electrical energy to heat raw materials, it is possible to precisely control the progress of the oxidation process and the release of slags. In this case, it is important to ensure the appearance of toxins. They are a transmitter of oxygen and heat. This technology allows you to reduce the concentration of harmful substances, for example, phosphorus and sulfur. Electric smelting can take place in a wide variety of environments: excess pressure, vacuum, or a certain atmosphere. Conducted research indicates that electric steel is of the highest quality. Technology is used to produce high-quality high-alloy, corrosion-resistant, heat-resistant and other types of steel. To convert electrical energy into heat, a cylindrical arc furnace with a spherical bottom is used. To ensure the most favorable melting conditions, the internal space is finished using heat-resistant metal. The device can only operate when connected to a three-phase network. It is worth considering that the electrical supply network must withstand a significant load. The source of thermal energy is an electric arc that occurs between the electrode and the molten metal. Temperatures can be over 2000 degrees Celsius.
- Oxygen converter. Continuous casting of steel in this case is accompanied by active injection of oxygen, due to which the oxidation process is significantly accelerated. This manufacturing method is also used to produce cast iron. It is believed that this technology has the greatest versatility and allows the production of metals with various properties.
The methods for producing galvanized steel are not very different from those considered. This is due to the fact that the change in the qualities of the surface layer occurs through chemical-thermal treatment.
There are other steel production technologies that are highly efficient. For example, methods based on the use of vacuum induction furnaces, as well as plasma arc welding.
Digital library
General technical disciplines / Production technology / Steel production in electric furnaces
Steel production in electric furnaces is increasing from year to year. They can produce a higher temperature and a reducing or neutral atmosphere, which is very important when smelting high-alloy steels.
For steel production, three-phase electric arc furnaces (Fig. 6.4) with vertical graphite or carbon electrodes and a non-conductive hearth are most often used. The current that heats the bath in these furnaces passes through the circuit electrode - arc - slag - metal - slag - arc - electrode. The capacity of such furnaces reaches 400 tons.
The furnace (Fig. 6.4) consists of a cylindrical metal casing and a spherical or flat bottom. The inside of the furnace is lined with refractory materials. Like open-hearth furnaces, arc furnaces can be acidic or basic. In the main furnaces, the hearth is laid out of magnesite brick, on top of which a packed layer of magnesite or dolomite 150 - 200 mm thick is made. In acid furnaces, silica bricks and quartzite packing on liquid glass are used.
Depending on the composition of the processed raw materials, the grade of steel being smelted, as well as the design and material of the furnace lining, the melting process can vary greatly. As an example, let's briefly look at steel melting with oxidation in a main arc furnace.
Such melting is typical for the case when the processed raw material contains phosphorus and differs significantly in the composition of other elements from the composition of a given steel grade. After loading the furnace, the electrodes are lowered onto the metal charge, having previously covered it on top with lime in an amount of 2 - 3% of the weight loaded into
metal furnace. Lime promotes smooth arc burning, protects materials from absorbing gases and forms slag faster. Melting is carried out at the highest furnace power in order to quickly create a liquid phase in the furnace.
Even before the complete melting of the charge, lime and iron ore are poured into the furnace to produce oxidizing slag in the first smelting period.
10 - 15 minutes after loading the ore, 60 - 70% of the slag is downloaded from the furnace - most of the phosphorus is removed with it.
After downloading the slag, lime is again poured into the furnace, the metal is completely melted and heated, and iron ore and lime are poured in portions. As the temperature rises, the oxidation of carbon and boiling of the bath intensify, which, as is known, helps remove gases and non-metallic inclusions dissolved in it from the metal.
During the boiling period, to completely remove phosphorus from the metal, the slag is downloaded several times. Instead of the downloaded slag, a new one is sent.
After the carbon content in the metal reaches the lower limit for a given grade, and the phosphorus content drops to 0.015%, the slag is again removed and the bath is allowed to “boil” for 25 minutes, without adding ore. After this, the recovery period of smelting begins. A mixture of lime, fluorspar CaF2 and fine coke is charged into the furnace to form a reducing slag. The iron and manganese oxide present in the bath metal begins to pass into the slag and is reduced by the coke carbon present in the slag. After the slag has been whitened, even stronger reducing agents are introduced into it - ground ferrosilicon or aluminum.
A distinctive feature of steel smelting in electric furnaces is the active deoxidation of slag, which leads to diffusion deoxidation of the metal, continuously releasing oxygen dissolved in it into the reducing slag. This deoxidation method prevents contamination of the metal with non-metallic inclusions released during deoxidation.
The duration of steel smelting in an arc furnace is 6–8 hours and depends on its power and design, the grade of steel being smelted, as well as the nature of the feedstock.
Electricity consumption depends on the power and design of the furnace, the duration of smelting and, consequently, the nature of the raw material and the given grade of steel. To smelt carbon steel weighing 1 ton, 500 – 700 kWh/t is consumed.
Open hearth method
The essence of this technology is the processing of cast iron and other scrap metal using a reverberatory furnace. The production of various steels in open hearth furnaces can be characterized by the fact that the charge is exposed to high temperatures. To supply high temperature, various fuels are burned.
Scheme of an open hearth furnace
Considering the open-hearth method of steel production, we note the following points:
- Open hearth furnaces are equipped with a system that supplies heat and removes combustion products.
- Fuel is fed into the combustion chamber alternately, from the right and then from the left. This ensures the formation of a torch, which leads to an increase in the temperature of the working environment and its maintenance for a long period.
- At the time of loading the charge, a sufficiently large amount of oxygen enters the combustion chamber, which is necessary for the oxidation of iron.
When producing steel using the open-hearth method, the holding time of the charge is 8-16 hours. Throughout the entire period, the oven operates continuously. Every year the design of the furnace is improved, which makes it possible to simplify the steel production process and produce metals of various qualities.
Charge materials
§ 34.
Metals, alloys, special alloys, slag-forming additives and other materials that are used to prepare various alloys are called charge materials or charge in the foundry industry. The charge includes: fresh materials (blast furnace cast iron of various grades, copper, aluminum, zinc, nickel, etc.), which come to foundries from metallurgical plants; scrap of ferrous alloys and scrap of non-ferrous alloys, which are processed industrial wastes; special ferroalloys and alloys (intermediate alloys of more refractory elements with low-melting elements) coming from metallurgical plants; waste from foundries and machine shops (sprues, profits, defective parts and briquetted shavings). The quantitative ratio of various materials in the charge depends on the quality of the starting materials and on the requirements for the manufactured alloys.
§ 35.
Main types of melting furnaces
In foundry production, melting furnaces operating on solid, liquid or gaseous fuels (coke, oil, fuel oil, gas) and electric furnaces are used. The first type of furnaces includes cupola furnaces and crucible furnaces, the second type includes electric arc furnaces and electric induction furnaces. The most widely used furnaces for melting cast iron are shaft-type furnaces—cupola furnaces. Gray cast iron produced in these furnaces is used for casting parts of various complexity. Electric furnaces melt steel, alloy cast iron, and white cast iron, which is then processed into malleable cast iron. The cupola furnace diagram is shown in Fig. 35. The cupola is a shaft furnace, the basis of which is a welded metal casing 1, lined on the inside with refractory bricks 2. The gap between the casing and the lining is filled with dry quartz sand 3. There is a loading window in the upper part of the cupola 4. Part of the cupola shaft located below the loading window , is lined with cast iron hollow bricks 5, which protect it from destruction when loading the charge 7.
Load the cupola using a skip hoist or jib crane. The upper part of the cupola ends with spark arrester 6.
To maintain combustion in the cupola furnace, air (blown) is supplied through special holes 8, called tuyeres, and blown by a fan. Molten cast iron along the hearth 9, located in the lower part of the shaft, flows through a special hole and chute into the reservoir 10. At the beginning of work, a layer of coke 500-1500 mm high is loaded into the cupola and ignited. This layer of coke is called blank shell. Then the working coke shell, flux and the first portion of the metal charge are loaded onto the idle shell. After loading the materials, the air necessary for combustion of the fuel is supplied through the tuyeres. In the melting belt, cast iron and slag are melted and flow into the cupola furnace. The resulting gases, rising upward, heat the metal charge and fuel, and then go into the pipe.
As the coke burns and the cast iron melts, the charge loaded into the cupola falls down, and new portions of charge materials are loaded in its place. During the melting process, liquid iron accumulates in the cupola furnace. The slag floats to the surface of the cast iron and is periodically released through the slag tap hole. The accumulated cast iron is drained through a tap hole along a chute into a special storage tank, and then into a ladle. The productivity of cupola furnaces is 0.5–30 tons of cast iron per hour.
For the purposes of fire safety and protection from pollution of the surrounding area, cupola furnaces are equipped with spark arresters, which are also dust collectors.
For melting steel, foundries use open-hearth and electric arc furnaces with basic and acid linings, as well as induction crucible furnaces.
In Fig. Figure 36 shows a diagram of an electric arc furnace. The heat source in this furnace is an electric arc that occurs between the melt 3 located in the bath of the furnace 4 and three graphite electrodes 1 (passing through the roof of the furnace 6), through which an electric current is supplied. The capacity of such furnaces is 1.5-10 tons. Melting duration is 1.5-4 hours. The prepared metal is poured when the furnace is tilted, carried out by a special mechanism, through chute 5. The charge is loaded into the furnace through window 2 or through the furnace roof, raised and rotated by a special mechanism.
Melting steel in an electric arc furnace consists of the following operations: filling the electric furnace, filling the charge, melting the charge and casting the finished steel.
An induction furnace (Fig. 37) consists of a frame 6 made of non-magnetic material, inside of which there is an inductor (coil) made of turns 7 of a copper tube through which cooling water flows. The turns are separated from each other by insulation '8. The melting crucible 5 in this furnace is made of a stuffed lining. The upper parts of the lining 1 and 3 and its lower part 4 are made of shaped refractory bricks, layer 2 is made of refractory coating. The heat source in these furnaces is an induction current excited in the charge loaded into the crucible when an alternating current of high frequency is passed through an inductor.
Rice. 38. Single-phase electric arc furnace for melting copper alloys
Melting of non-ferrous alloys is carried out in crucible furnaces with oil or gas heating, in electric resistance furnaces, as well as in arc or induction electric furnaces.
Copper alloys are melted in crucible, flame and electric furnaces. The most widely used are single-phase electric arc furnaces of the DMK type (Fig. 38). The furnace is a metal drum 1, lined with refractory bricks 2. The heat required to melt copper is created by an electric arc arising between two horizontally located electrodes 3. Mounted on rollers 4, drum 1 can be rotated to a certain angle by a motor and a gear drive.
Charge materials are loaded through a working window equipped with a chute through which the finished melt is released. For the smelting of copper alloys, the charge materials are pigs, machine scrap, waste from own production, etc. During the smelting process of copper, zinc and lead, harmful gases and vapors are released, so smelting furnaces are equipped with powerful exhaust ventilation.
Aluminum alloys are melted in crucible and flame furnaces, electric resistance furnaces and induction furnaces. In Fig. 39 shows a gas-heated crucible furnace for melting aluminum alloys. The furnace has a refractory lining 1, inside which a cast iron crucible 2 is inserted. Gas is supplied to the burner 4 and burns in the space between the lining and the crucible. Hole 3 is provided for releasing metal when the crucible burns out. Combustion products and gases from the alloy are removed by an exhaust hood 5. The furnace is suspended by axles on side supports and can be tilted using a steering wheel and a worm gear.
In oxygen converters
Today, various steels are produced in oxygen converters. This technology involves purging liquid cast iron in a converter. To do this, pure oxygen is supplied. The features of this technology include the following points:
- A converter is a special equipment, which is represented by a pear-shaped steel vessel. The capacity of such a device is 100-350 tons. The inside of the structure is lined with refractory bricks.
- The design of the upper part includes a neck, which is necessary for loading the charge and liquid cast iron. In addition, gases formed during the melting of raw materials are removed through the neck.
- Pouring cast iron and adding other charge is carried out at a temperature of about 1400 degrees Celsius. In order to ensure active oxidation of iron, pure oxygen is supplied under a pressure of about 1.4 MPa.
- When a large amount of oxygen is supplied, cast iron and other mixtures oxidize, which causes the release of a large amount of heat. Due to strong heating, the entire charge material melts.
- At the moment when excess carbon is removed from the composition, the blowing stops and the lance is removed from the converter. Typically, purging continues for 20 minutes.
- At this stage, the resulting composition contains a large amount of oxygen. That is why, to improve performance, various deoxidizing agents and alloying elements are added to the composition. The resulting slag is removed into a special slag ladle.
- Converter melting time may vary, as a rule, it is 35-60 minutes. The holding time depends on the type of charge used and the volume of steel produced.
Read also: What is the name of a drilling machine?
It is worth considering that the productivity of such equipment is about 1.5 million tons with a capacity of 250 tons. This technology is used to produce carbon, low-carbon, and alloy steels. The oxygen-converter method of steel production was developed quite a long time ago, but today it is still very popular. This is due to the fact that when using this technology, high-quality metals can be obtained, and the productivity of the technology is very high.
In conclusion, we note that it is almost impossible to produce steel at home. This is due to the need to heat the charge to a sufficiently high temperature. At the same time, the process of iron oxidation is very complex, as is the removal of harmful impurities
If you find an error, please select a piece of text and press Ctrl+Enter.
Electric furnaces have the following advantages:
- • fast heating and temperature maintenance up to 2000 °C;
- • the ability to create an oxidizing, reducing, neutral environment, as well as high vacuum, which leads to more complete deoxidation of steel;
- • introduction of high-calcareous slags (due to high temperature), which allows for more complete removal of sulfur and phosphorus;
- • accuracy of obtaining steel of a given composition.
Electric furnaces are used for smelting high-quality carbon, alloy, high-alloy, tool steels and heat-resistant alloys.
According to the principle of operation, electric furnaces are divided into arc and induction. The productivity of arc furnaces is 0.6-200 tons, induction furnaces - 0.6-60 tons. In such furnaces it is possible to smelt metals with a high concentration of refractory elements, such as chromium, tungsten, nickel, cobalt, etc.
In an electric arc furnace
(Fig. 1.7) an electric arc is formed between the graphite electrodes
4
and the charge.
At the same time, high power (16,000 kW) is developed in a relatively small volume. In the arc plasma the temperature reaches 10,000 °C and higher. The furnace is loaded through window 6,
and the finished metal is discharged through tap hole
3
and chute
2.
To tilt and drain metal and slag, the furnace is equipped with rollers/electric drive.
Steel scrap, iron ore (to oxidize impurities) and fluxes are loaded into electric furnaces, and alloying additives (ferromanganese, ferrosilicon, etc.) are introduced for deoxidation. In order to save energy and increase productivity, cast iron is rarely used.
Rice. 1.7.
Electric arc furnace:
1
- rollers;
2
- gutter;
3
- tap hole;
4
-
electrode; 5 - vault; 6
- loading window
When electric smelting, oxygen purging is often used, as a result of which the furnace productivity increases by 20-30%, and expensive ferroalloys, electrodes, and electricity are saved.
Melting in induction furnaces
characterized by low waste of alloying elements, high efficiency, and the ability to accurately control temperature. In addition, compared to electric arc furnaces, induction furnaces do not have electrodes or electric arcs, which makes it possible to produce steels with a low carbon and gas content. The disadvantages of these furnaces include the low temperature of the slag, which does not allow phosphorus and sulfur to be removed during melting as intensively as in arc furnaces, and the low durability of the lining.
Induction furnaces are usually used for smelting high-alloy steels and special-purpose alloys (corrosion-resistant, heat-resistant, etc.).
There are large furnaces (up to 25 tons) operating at industrial frequency current (50 Hz), as well as medium and small ones (up to 60 kg). The furnaces are powered by current generators with a frequency of 500 to 2500 Hz.
In Fig. Figure 1.8 shows the structure of an induction furnace for melting steel. Inside a spiral multi-turn inductor 4
, made of a copper tube in which water circulates for cooling, there is a fireproof crucible
5 .
Rice. 1.8.
Induction electric furnace:
1
- metal;
2
- removable arch;
3
- axis;
4
- water-cooled inductor; 5 - crucible; 6 — body; 7 - high generator
The supply generator is connected to the inductor terminals. The inductor and crucible are installed in the housing 6.
To produce finished steel, the entire furnace is rotated around axis
3.
When current is passed through inductor
4,
the metal in the crucible is in a rapidly alternating electromagnetic field and is heated by induced currents.
To produce steels with a minimum content of gases and non-metallic inclusions, induction furnaces are used for melting in a rarefied environment (in a vacuum) and in a protective gas environment (for example, argon).
Electric melting furnaces have advantages compared to other melting units:
a) it is easy to regulate the thermal process by changing current parameters;
b) it is possible to obtain a high temperature of the metal,
c) the ability to create an oxidizing, reducing, neutral atmosphere and vacuum, which allows the metal to be deoxidized with the formation of a minimum amount of non-metallic inclusions.
Electric furnaces are used for smelting structural, high-alloy, tool, and special alloys and steels.
There are arc and induction electric furnaces.
Arc melting furnace.
Rice. 5. Diagram of an arc melting furnace
The arc furnace is powered by three-phase alternating current. Has three cylindrical electrodes 9
from a graphitized mass, fixed in electrode holders
8
, to which electric current is supplied via cables
7
.
An electric arc occurs
between the electrode and the metal charge 3 The furnace body has the shape of a cylinder. Outside it is enclosed in a durable steel casing 4
, inside it is lined with basic or acid brick
1
.
The melting space is limited by walls 5
, hearth
12
and roof
6.
The removable roof
6
has holes for electrodes.
In the wall of the housing there is a working window 10
(for draining slag, loading ferroalloys, taking samples), closed during melting with a shutter.
The finished steel is discharged through a drain hole with a drain chute 2
.
The furnace rests on sectors and has a drive 11
for tilting towards the working window or chute. The furnace is loaded with the roof removed.
The capacity of the furnaces is 0.5…400 tons. In metallurgical shops, electric furnaces with a basic lining are used, and in foundries - with an acid lining.
Read also: Cleaning brass with citric acid
In the main arc furnace, two types of smelting are carried out:
a) on a charge from alloyed waste (by remelting method),
b) on a carbonaceous charge (with oxidation of impurities).
Melting on a charge made from alloyed waste
conduct without oxidation of impurities. After melting the charge, sulfur is removed from the metal, introducing the main slag, if necessary, it is carburized and the metal is brought to the specified chemical composition. Diffusion deoxidation is carried out by feeding crushed ferrosilicon, aluminum, and ground coke onto the slag. This is how alloy steels are smelted from waste from machine-building plants.
Melting on a carbon charge
used for the production of structural steels. The furnace is loaded with the following mixture: steel scrap, pig iron, scrap electrodes or coke for carburizing metals and lime. The electrodes are lowered and the current is turned on. The charge melts under the action of the electrodes, and the metal accumulates in the bottom of the furnace. During the melting of the charge, air oxygen, charge oxides and scale oxidize iron, silicon, phosphorus, manganese, and partially carbon. Calcium oxide from lime and iron oxide form the main ferrous slag, which helps remove phosphorus from the metal. After heating to 1500...1540 0 C, ore and lime are loaded, a period of “boiling” of the metal is carried out, and further oxidation of carbon occurs. After boiling stops, the slag is removed. Then they begin to remove sulfur and deoxidize the metal of a given chemical composition. Deoxidation is carried out by precipitation and diffusion method. To determine the chemical composition of the metal, samples are taken and, if necessary, ferroalloys are introduced into the furnace to obtain the desired chemical composition. Then final deoxidation is performed with aluminum and silico-calcium, and the steel is released into the ladle.
When smelting alloy steels in arc furnaces, alloying elements in the form of ferroalloys are introduced into the steel.
High-quality carbon steels - structural, tool, heat-resistant and heat-resistant - are smelted in arc furnaces.
Induction Crucible Melting Furnaces
They smelt the highest quality corrosion-resistant, heat-resistant and other steels and alloys.
Capacity from tens of kilograms to 30 tons.
Rice. 6. Diagram of an induction crucible furnace
The furnace consists of a water-cooled inductor 3
, inside of which there is a crucible
4
(basic or acidic refractory materials) with a metal charge, a single-phase alternating current of high frequency (500...2000 Hz) passes through the inductor from a high-frequency generator.
When passing current through an inductor in metal 1
located in the crucible, powerful eddy currents are induced, which ensures heating and melting of the metal. To reduce heat loss, the stove has a removable roof
2
.
The crucible is made from acidic (quartzite) or basic (magnesite powder) refractories. To release the melt, the furnace is tilted towards the drain chute.
Under the influence of the electromagnetic field of the inductor during melting, intensive circulation of liquid metal occurs, which helps to accelerate chemical reactions, obtain a metal with a homogeneous chemical composition, quickly float up non-metallic inclusions, and equalize the temperature.
In induction furnaces, steel and alloys are smelted from alloyed waste by remelting
, or from pure charge iron and scrap with the addition of ferroalloys
by fusion
.
After melting the charge, a slag mixture is loaded onto the surface of the metal to reduce heat losses of the metal and reduce the loss of alloying elements, protecting it from saturation with gases.
When smelting in acid furnaces, after melting and removing the smelting slag, slag is removed from broken glass. For final deoxidation, ferrosilicon, ferromanganese and aluminum are introduced into the ladle before releasing the metal.
In the main furnaces, deoxidation is carried out with a mixture of powdered lime, coke, ferrosilicon, ferromanganese and aluminum.
In the main furnaces, high-quality alloy steels with a high content of manganese, titanium, nickel, and aluminum are smelted, and in acid-lined furnaces, structural steels alloyed with other elements are smelted.
In furnaces it is possible to produce steels with low carbon content and carbon-free alloys, since there is no carburizing medium.
In vacuum induction melting, the inductor, crucible, batch dispenser and molds are placed in vacuum chambers. High-quality alloys with a low content of gases, non-metallic inclusions and alloys alloyed with any elements are obtained.
Steel casting.
From the melting furnaces, steel is released into a ladle, which is carried by an overhead crane to the steel casting site. From the ladle, the steel is poured into the molds or molds of a continuous casting machine. In molds or crystallizers, steel hardens and ingots are obtained, which are rolled and forged.
Molds
– cast iron molds for the production of ingots.
Molds are made with square, rectangular, round and multifaceted cross sections.
Ingots with a square cross-section are converted into long products: I-beams, channels, angles. Rectangular ingots - into sheets. Round ingots are used to make pipes and wheels. Ingots with a multifaceted cross-section are used for the manufacture of forgings.
Calm and boiling carbon steels are poured into ingots weighing up to 25 tons, alloy and high-quality steels into ingots weighing 0.5...7 tons, and some types of high-alloy steels into ingots weighing up to several kilograms.
Steel is poured into molds from above (Fig. 7.a), from below (by siphon) (Fig. 7.b) and on continuous casting machines (Fig. 7).
In the molds from above
steel is poured directly from ladle
1
. This eliminates the consumption of metal on the gates and simplifies the preparation of equipment for casting. The disadvantages include the poorer quality of the surface of the ingots, due to the presence of oxide films from metal splashes that harden on the walls of the mold.
Used for casting carbon steels.
Fig.7. Pouring steel into molds
a – from above; b – from below (siphon)
For siphon casting
Several molds (4…60) are filled simultaneously.
The molds are installed on a pallet 6
, in the center of which there is a center sprue
3,
lined with refractory tubes
4
, connected by channels
7
to the molds.
Liquid steel 2
from ladle
1
enters the center sprue and smoothly, without splashing, fills the mold
5
.
The surface of the ingot is clean, and a large mass of metal can be poured simultaneously into several molds.
Used for alloy and high-quality steels.
Continuous casting
steel is that liquid steel from ladle
1
through an intermediate casting device
2
is continuously fed into a water-cooled mold without a bottom - crystallizer
3
, from the bottom of which a solidifying ingot
5
.
Before pouring the metal into the crystallizer, a seed is introduced - a steel rod with a replaceable head having a dovetail-shaped groove, which at the beginning of pouring serves as the bottom of the crystallizer. Due to intense cooling, the liquid metal at the walls of the crystallizer and on the seed solidifies, and a crust forms, connecting the metal with the seed. The seed moves downwards using traction rollers 6
, gradually pulling the solidifying ingot out of the crystallizer.
After passing through the traction rollers 6
, the seed is separated.
The pulling speed is on average 1 m/min. Final hardening in the core occurs as a result of secondary cooling with water from the spray 4
.
Then the hardened ingot enters the cutting zone, where it is cut with a gas cutter 7
into pieces of a given length. The ingots have a dense structure and a fine-grained structure; there are no shrinkage cavities.
Rice. 8. Scheme of continuous steel casting
Read also: Pinout of utp cable 8 cores
Ways to improve the quality of steel
The quality of metal can be improved by reducing harmful impurities, gases, and non-metallic inclusions in it. To improve the quality of metal, they use: treatment with synthetic slag, vacuum degassing of metal, electroslag remelting (ESR), vacuum arc remelting (VAR), metal remelting in electron arc and plasma furnaces, etc.
Vacuum degassing
carried out to reduce the content of gases and non-metallic inclusions in the metal.
Vacuuming
steel is carried out in a ladle, when pouring from ladle to ladle, when pouring into a mold.
To vacuum the ladle, the ladle with liquid steel is placed in a chamber closed with a sealed lid. Vacuum pumps create a vacuum to a residual pressure of 0.267...0.667 kPa. When the pressure decreases, hydrogen and nitrogen are released from the liquid steel. The floating gas bubbles capture non-metallic inclusions, as a result of which their content in the steel decreases. The strength and ductility of steel improves.
Electroslag remelting (ESR))
used for smelting high-quality steels for bearings and heat-resistant steels.
Metal melted in an arc furnace and rolled into a rod is subjected to remelting. The heat source is a slag bath heated by electric current. Electric current is supplied to the electrode being melted 1
immersed in a slag bath
2
, and to a tray
9
installed in a water-cooled crystallizer
7
, which contains a seed
8
.
The released heat heats bath 2
to a temperature above 1700? C and causes melting of the end of the electrode.
Drops of liquid metal 3
pass through the slag and form a metal bath
4
. The transfer of metal droplets through the main slag helps remove sulfur, non-metallic inclusions and gases from the metal. The metal bath is replenished by melting the electrode, and under the influence of the crystallizer it is gradually formed into ingot 6. The oxygen content decreases by 1.5...2 times, sulfur by 2...3 times. The ingot is distinguished by density, uniformity, good surface quality, and high mechanical and performance properties. Ingots are produced in round, square and rectangular sections, weighing up to 110 tons.
Fig.9. Electroslag remelting circuit
Vacuum arc remelting (VAR)
used to remove gases and non-metallic inclusions from metal.
The process is carried out in vacuum arc furnaces with a consumable electrode. The cathode is produced by mechanical processing of an ingot smelted in electric furnaces or ESR installations.
Fig. 10. Scheme of vacuum-arc remelting
Consumable electrode 3
fixed on a water-cooled rod
2
and placed in the furnace body
1
and then in a copper water-cooled mold
6
.
Air is pumped out of the furnace body to a residual pressure of 0.00133 kPa. When voltage is applied between the consumable electrode 3
(cathode) and the seed
8
(anode), an arc occurs.
The generated heat melts the end of the electrode. Drops of liquid metal 4
, passing through the arc discharge zone, are degassed, fill the mold and solidify, forming an ingot
7
.
The arc burns between the electrode and liquid metal 5
in the upper part of the ingot throughout the entire melting process. Cooling the ingot and heating the liquid metal creates conditions for directed solidification of the ingot. Consequently, non-metallic inclusions are concentrated in the upper part of the ingot, and the shrinkage cavity is small. The ingot is characterized by high uniformity of chemical composition and improved mechanical properties. They produce parts for turbines, engines, and aircraft structures. The weight of the ingots reaches 50 tons.
There are many criteria by which steels can be classified, therefore the generally accepted criteria for classifying steels are: chemical composition, purpose, structure and quality of steels.
By chemical composition
Steels are classified into carbon and alloy.
Carbon
steels are divided into
A positive feature of carbon steels is a fairly high set of mechanical properties that are achieved by heat treatment and good technological properties: cutting machinability, weldability and pressure machinability. They became quite cheap.
The main disadvantages of carbon steels are low hardenability, therefore the set of properties achieved during heat treatment can only be achieved in parts with a small cross-section (6-12 mm). Steels are sensitive to overheating during heat treatment, which determines the tendency for austenite grain growth. The need to use sharp coolants during hardening leads to increased stress and increased defects. Therefore, carbon steels are used for the manufacture of simple parts with small cross-sections.
Alloy steels are steels with the addition of elements of other metals, called alloying elements. Depending on the elements introduced, steels are divided into chromium, manganese, chromium-nickel, etc. The total content of alloying elements should be no more than 3-5% in low-alloy steels and more than 10% in high-alloy steels.
The introduction of alloying additives into steel significantly increases the resistance to plastic deformation after heat treatment compared to carbon steels with equal carbon content. Alloying elements provide increased hardenability of steel, reduced warping and cracks. By regulating the amount and composition of alloying additives, a certain property of steel is achieved.
By purpose
steels are divided into structural and instrumental.
Structural steel
used for the manufacture of machine parts, structures in mechanical engineering and construction. The main requirement for structural steel is to provide rigidity, static and cylindrical strength with guaranteed reliability and durability.
The high modulus of elasticity (E = 2.1x10 6 MPa) provides high rigidity and determines its use for the manufacture of building structures and body parts, shafts, etc.
Structural steels are divided into: construction, engineering and steels with special properties (heat-resistant, heat-resistant, corrosion-resistant).
Tool steels
apply:
– for the manufacture of tools for cutting materials with a high carbon content (from 0.7 to 1.2%);
– dies for cold and hot deformation
and must have high hardness, strength, wear resistance and a number of other properties.
The quality of steel is classified
| R | S | ||
| Standard quality steel | No more | 0,045% | 0,055% |
| Quality steel | No more | 0,035% | 0,040% |
| High quality steel | No more | 0,025% | 0,025% |
| Extra high quality steel | No more | 0,025% | 0,015% |
Steel marking
Our country has adopted an alphanumeric system for designating steel and alloy grades.
Carbon structural quality steels are designated by a two-digit number indicating the average carbon content in hundredths of a percent.
For tool carbon steels, the letter “U” and the carbon content in tenths of a percent (U7, U9, etc.) are entered into the steel grade.
In alloyed steels, the presence of one or another alloying element is indicated in the grade by a letter that conventionally corresponds to each element. After the letter, the quantity of this element is indicated, rounded to a whole number. If the alloying element present in the steel is less than 1.5%, then the number is not placed after the index.
If the carbon content in alloy carbon steel is 1% or more, then the number is not put at the beginning of the mark (ХГСВ).
In high-speed steel grades, the first letter “P” was introduced. and then indicate the amount of tungsten as a percentage. All high-speed steels contain about 4% chromium, so the letter “X” is not indicated in the brand designation. And the amount of carbon in steel is also not indicated, because its amount is proportional to the amount of vanadium. (P18, P6M5).
did not you find what you were looking for? Use the search:
Construction of electric furnaces for metal smelting
Electric arc smelting of metal is carried out in an electric furnace with a capacity of 0.5 to 200 tons. Furnaces with capacities of 300 and 400 tons are being developed. The schematic diagram of an electric arc furnace is shown in Fig. 1.
The furnace body has the shape of a cylinder with a spherical or flat bottom. Externally, it has a protective casing made of steel sheet with a thickness of 10...40 mm, the internal surface is lined with basic or acidic refractories. Carbon or graphite electrodes are passed through holes in the furnace roof. There is a working window in the wall of the housing through which slag is drained, ferroalloys are loaded, and metal samples are taken.
Two types of steel-smelting electric furnaces are used: arc and high-frequency induction. Arc furnaces, in which charge materials are melted by the heat of an electric arc, are the most common due to their high efficiency, the ability to smelt steel of various grades, simplicity of structure and ease of maintenance.
Furnaces are lined with basic or acidic refractory materials. Furnaces with a main lining are more common, since they can remove sulfur and phosphorus from liquid steel. Modern electric arc furnaces are equipped with special devices to supply oxygen, which is used to oxidize impurities during steel melting.
Technology of steel melting in the main electric arc furnace
Depending on the composition of the charge, steel can be melted in electric furnaces with a main lining using three methods:
- with complete oxidation of impurities,
- with their partial oxidation,
- without oxidation.
Materials scientist
Cast iron is converted into steel in metallurgical units of various operating principles: open-hearth furnaces, oxygen converters, electric furnaces.
Steel production in open hearth furnaces
Martin process (1864-1865, France). For the first time, after numerous attempts, it was possible to obtain liquid steel on the hearth of a fiery furnace, since previously steel in a dough-like state was obtained in this way. Marten applied the principle of heat recovery from waste furnace gases to a steel-smelting furnace to heat the fuel and air supplied to the furnace. Until the seventies of the twentieth century, it was the main method of steel production. The method is characterized by relatively low productivity and the possibility of using secondary metal – steel scrap. The capacity of the furnace is 200…900 tons. The method makes it possible to produce high-quality steel.
The open hearth furnace (Fig. 3) in its design and principle of operation is a flame reverberatory regenerative furnace. Gaseous fuel or fuel oil is burned in the smelting space. The high temperature for obtaining steel in a molten state is provided by heat recovery from furnace gases.
A modern open-hearth furnace is a horizontally elongated chamber made of refractory brick. The working melting space is limited from below by the hearth 12, from above by the arch 11, and from the sides by the front 5 and rear 10 walls. The hearth has the shape of a bathtub with slopes towards the walls of the furnace. In the front wall there are loading windows 4 for supplying charge and flux, and in the rear wall there is a hole 9 for releasing finished steel.
Open hearth furnace
Rice. 3. Scheme of an open hearth furnace
A characteristic of the working space is the area of the furnace bottom, which is calculated at the level of the thresholds of the loading windows. The vaults are made of heat-resistant chromium-magnesite brick, which allows it to be heated to 1800 0C. Hot gas is supplied to the furnace through the central channel, air - through two side channels. Therefore, at both ends of the melting space there are furnace heads 2, which serve to mix fuel with air and supply this mixture into the melting space. Natural gas and fuel oil are used as fuel.
To heat air and gas when operating on low-calorie gas, the furnace has two regenerators 1.
Regenerator - a chamber in which a nozzle is placed - a refractory brick lined in a cage, which is designed to heat air and gases.
The gases leaving the furnace have a temperature of 1500...1600 0C. Entering the regenerator, the gases heat the nozzle to a temperature of 1250 0C. Air is supplied through one of the regenerators, which, passing through the nozzle, is heated to 1200 0C and enters the furnace head, where it is mixed with fuel. At the exit from the head, a torch 7 is formed, directed towards the charge 6.
The exhaust gases pass through the opposite head (left), cleaning devices (slag tanks), which serve to separate slag and dust particles from the gas, are sent to the second regenerator.
Cooled gases leave the furnace through chimney 8.
After cooling, the nozzles of the right regenerator switch the valves, and the flow of gases in the furnace changes direction.
The temperature of the flame reaches 1800 0C. The torch heats the furnace working space and the charge. The torch promotes the oxidation of charge impurities during smelting.
The duration of melting is 3...6 hours, for large furnaces - up to 12 hours. The finished melt is released through a hole located in the rear wall at the lower level of the hearth. The hole is tightly plugged with low-sintering refractory materials, which are knocked out when the melt is released. The furnaces operate continuously until they are stopped for major repairs - 400...600 heats.
Depending on the composition of the charge used in smelting, there are different types of open-hearth process:
– scrap process, in which the charge consists of steel scrap (scrap) and 25...45% pig iron, the process is used in factories where there are no blast furnaces, but a lot of scrap metal.
– scrap-ore process, in which the charge consists of liquid cast iron (55...75%), scrap and iron ore (15...30% by weight of the metal part of the charge). Iron ore is added to accelerate the oxidation of cast iron impurities. The process is used in metallurgical plants that have blast furnaces.
The furnace lining can be basic or acidic. If basic oxides predominate in the slag during steel melting, the process is called basic open-hearth process, and if acidic, it is called acidic.
The largest amount of steel is produced by the scrap ore process in open hearth furnaces with a main lining.
Iron ore and limestone are loaded into the furnace, and after heating, scrap is fed. When loading solid materials into the furnace, the maximum amount of fuel is consumed to ensure rapid heating and melting of the charge materials. After heating the scrap, liquid cast iron is poured into the furnace. During the melting period, due to ore oxides and scrap, cast iron impurities are intensively oxidized: silicon, phosphorus, manganese and partially carbon. The oxides form a slag with a high content of iron and manganese oxides (iron slag). After this, a period of “boiling” of the bath is carried out: iron ore is loaded into the furnace and the bath is purged with oxygen supplied through pipes 3. At this time, the supply of fuel and air to the furnace is turned off and the slag is removed.
To remove sulfur, new slag is created by applying lime with the addition of bauxite to the metal surface to reduce the viscosity of the slag. The CaO content in the slag increases, and FeO decreases.
During the “boiling” period, carbon is intensively oxidized, so the charge must contain excess carbon. At this stage, the metal is brought to a given chemical composition, gases and non-metallic inclusions are removed from it.
Then the metal is deoxidized in two stages. First, deoxidation occurs by oxidizing the carbon of the metal, with the simultaneous supply of deoxidizing agents - ferromanganese, ferrosilicon, aluminum - to the bath. The final deoxidation with aluminum and ferrosilicon is carried out in a ladle when the steel is released from the furnace. After taking control samples, the steel is released into the ladle.
In the main open-hearth furnaces, low- and medium-alloy carbon structural steels (manganese, chromium) are smelted, in addition to high-alloy steels and alloys, which are produced in electric melting furnaces.
In acid open-hearth furnaces, the slag is acidic and does not contain free lime. Consequently, the removal of sulfur and phosphorus does not occur in such a furnace, so a charge with a low content of sulfur and phosphorus is used. They smelt high-quality steels and high-quality alloy steels. Steels contain less hydrogen and oxygen and non-metallic inclusions. Consequently, acid steel has higher mechanical properties, especially impact strength and ductility, and is used for particularly critical parts: crankshafts of large engines, rotors of powerful turbines, ball bearings.
The main technical and economic indicators of steel production in open hearth furnaces are:
– furnace productivity – steel removal from 1 m2 of hearth area per day (t/m2 per day), on average 10 t/m2;
– fuel consumption per 1 ton of steel produced is on average 80 kg/t.
As furnaces become larger, their economic efficiency increases.
Watch the video “Smelting steel in Mrtenov furnaces.”
Steel production in oxygen converters
The oxygen-converter process is the smelting of steel from liquid cast iron in a converter with a main lining and blowing oxygen through a water-cooled lance.
The first experiments in 1933-1934 - Mozgovoy.
On an industrial scale - in 1952-1953 at factories in Linz and Donawitz (Austria) - it was called the LD process. Currently, the method is the main one in the mass production of steel.
An oxygen converter is a pear-shaped vessel made of steel sheet, lined with base brick.
Converter capacity is 130...350 tons of liquid cast iron. The converter is mounted in a cast steel ring that has two axles, with which it rests on the bearings of two racks, so during operation the converter can be rotated 360° to load scrap, pour cast iron, drain steel and slag.
The charge materials of the oxygen-converter process are liquid pig iron, steel scrap (no more than 30%), lime for slag removal, iron ore, as well as bauxite Al2O3 and fluorspar CaF2 for liquefying the slag.
The sequence of technological operations when melting steel in oxygen converters is presented in Fig. 4.
After the next steel melting, the outlet hole is sealed with a refractory mass and the lining is inspected and repaired.
Before melting, the converter is tilted, scrap is loaded using charging machines (Fig. 4, a), cast iron is poured at a temperature of 1250...1400 0C (Fig. 4, b).
The sequence of technological operations when melting steel in an oxygen converter
Rice. 4. Sequence of technological operations when melting steel in oxygen converters
After this, the converter is turned to the working position (Fig. 4, c), a cooled lance is inserted inside and oxygen is supplied through it at a pressure of 0.9...1.4 MPa. The lance does not reach the metal level by 1200...1400 mm, so oxygen is supplied to the surface of the metal poured into the converter, and is not blown under the metal mirror (like air in previously used converters). Simultaneously with the start of blowing, lime, bauxite, and iron ore are loaded. Oxygen penetrates the metal, causing it to circulate in the converter and mix with the slag. A temperature of 2400 0C develops under the tuyere. In the zone of contact of the oxygen jet with the metal, iron is oxidized. Iron oxide dissolves in the slag and metal, enriching the metal with oxygen. Dissolved oxygen oxidizes silicon, manganese, and carbon in the metal, and their content decreases. The metal is heated by the heat released during oxidation.
Phosphorus is removed at the beginning of purging the bath with oxygen, when its temperature is low (the phosphorus content in cast iron should not exceed 0.15%). If the phosphorus content is high, to remove it, it is necessary to drain the slag and introduce a new one, which reduces the productivity of the converter.
Sulfur is removed throughout the entire melting process (the sulfur content in cast iron should be up to 0.07%).
The oxygen supply is stopped when the carbon content in the metal corresponds to the specified value. After this, the converter is turned and the steel is released into a ladle (Fig. 4, d), where it is deoxidized by the precipitation method with ferromanganese, ferrosilicon and aluminum, then the slag is drained (Fig. 4, e).
The disadvantage of the oxygen-converter method of producing steel is the large dust formation caused by the abundant oxidation and evaporation of iron.
In oxygen converters, steels with different carbon contents, boiling and calm, as well as low-alloy steels are smelted. Alloying elements in molten form are introduced into the ladle before steel is released into it.
Melting in converters with a capacity of 130...300 tons ends in 25...30 minutes.
Watch the educational video "Technology of steel smelting in oxygen converters."
Steel production in electric furnaces
Electric melting furnaces have advantages compared to other melting units:
a) it is easy to regulate the thermal process by changing current parameters;
b) it is possible to obtain a high temperature of the metal,
c) the ability to create an oxidizing, reducing, neutral atmosphere and vacuum, which allows the metal to be deoxidized with the formation of a minimum amount of non-metallic inclusions.
Electric furnaces are used for smelting structural, high-alloy, tool, and special alloys and steels.
There are arc and induction electric furnaces.
Arc melting furnace
The arc furnace diagram is shown in Fig. 5. The arc furnace is powered by three-phase alternating current. It has three cylindrical electrodes 9 made of graphitized mass, fixed in electrode holders 8, to which electric current is supplied through cables 7. An electric arc occurs between the electrode and the metal charge 3. The furnace body has the shape of a cylinder. Outside, it is enclosed in a durable steel casing 4, inside it is lined with basic or acid brick 1. The melting space is limited by walls 5, a hearth 12 and a roof 6. The removable roof 6 has holes for electrodes. In the wall of the housing there is a working window 10 (for draining slag, loading ferroalloys, taking samples), closed during melting with a shutter. The finished steel is discharged through a drain hole with a drain chute 2. The furnace rests on sectors and has a drive 11 for tilting towards the working window for downloading slag or a chute for draining steel. The furnace is loaded with the roof removed.
The capacity of the furnaces is 0.5…400 tons.
Arc melting furnace diagram
Rice. 5. Diagram of an arc melting furnace
In metallurgical shops, electric furnaces with a basic lining are used, and in foundries - with an acid lining.
In the main arc furnace, two types of smelting are carried out:
a) on a charge from alloyed waste (by remelting method);
b) on a carbonaceous charge (with oxidation of impurities).
Melting on a charge made from alloyed waste is carried out without oxidation of impurities. The charge for such smelting should have less manganese and silicon than in the smelted steel, as well as a reduced phosphorus content. After melting the charge, sulfur is removed from the metal, introducing the main slag, if necessary, it is carburized and the metal is brought to the specified chemical composition. Diffusion deoxidation is carried out by feeding crushed ferrosilicon, aluminum, and ground coke onto the slag. This is how alloy steels are smelted from waste from machine-building plants.
Melting on a carbon charge is used for the production of structural carbon steels. Melting is carried out in two periods: oxidative and reduction.
The furnace is loaded with the following mixture: steel scrap, pig iron, scrap electrodes or coke for carburizing metals and lime. The electrodes are lowered and the current is turned on. The charge melts under the action of the electrodes, and the metal accumulates in the bottom of the furnace. During the melting of the charge, air oxygen, charge and scale oxides actively oxidize iron, silicon, phosphorus, manganese, and partially carbon. Calcium oxide from lime and iron oxide form the main ferrous slag, which helps remove phosphorus from the metal. After heating to 1500...1540 0C, ore and lime are loaded, a period of “boiling” of the metal is carried out, and further oxidation of carbon occurs. Boiling of a metal accelerates the removal of gases and non-metallic inclusions from it, and promotes the removal of phosphorus. Periodically, the slag is removed and ore and lime are added. When the carbon content becomes 0.1% less than the specified value, boiling is stopped. After boiling stops, the slag is removed.
During the recovery period of smelting, the metal is deoxidized with white slag (lime, fluorspar, coke and ferrosilicon) and the sulfur is removed and the metal is deoxidized to a given chemical composition. Deoxidation is carried out by precipitation and diffusion method. To determine the chemical composition of the metal, samples are taken and, if necessary, ferroalloys are introduced into the furnace to obtain the desired chemical composition. Then final deoxidation is performed with aluminum and silico-calcium, and the steel is released into the ladle.
When smelting alloy steels in arc furnaces, alloying elements in the form of ferroalloys are introduced into the steel.
High-quality carbon steels - structural, tool, heat-resistant and heat-resistant - are smelted in arc furnaces.
Induction Crucible Melting Furnaces
Induction melting furnaces produce the highest quality corrosion-resistant, heat-resistant and other steels and alloys that are subject to increased requirements.
Capacity - from tens of kilograms to 30 tons.
Induction furnaces can be equipped with systems to create a vacuum or controlled atmospheres.
Since in induction furnaces heat arises in the metal, the slag in them is heated only through the metal.
The diagram of an induction crucible furnace is shown in Fig. 6.
Diagram of an induction crucible furnace
Rice. 6. Diagram of an induction crucible furnace
The furnace consists of a water-cooled inductor 3, inside of which there is a crucible 4 (basic or acidic refractory materials) with a metal charge; a single-phase alternating current of high frequency (500...2000 Hz) passes through the inductor from a high-frequency generator.
When current is passed through the inductor in metal 1 located in the crucible, powerful eddy currents are induced, which ensures heating and melting of the metal. To reduce heat loss, the stove has a removable roof 2.
The crucible is made from acidic (quartzite) or basic (magnesite powder) refractories. To release the melt, the furnace is tilted towards the drain chute.
Under the influence of the electromagnetic field of the inductor during melting, intensive circulation of liquid metal occurs, which helps to accelerate chemical reactions, obtain a metal with a homogeneous chemical composition, quickly float up non-metallic inclusions, and equalize the temperature.
Melting steel from cast iron in induction furnaces has not become widespread, since oxidation and refining with slag is almost impossible in them.
In induction furnaces, steel and alloys are smelted from alloyed waste by remelting, or from pure charge iron and scrap with the addition of ferroalloys by fusion.
After melting the charge, a slag mixture is loaded onto the surface of the metal to reduce heat losses of the metal and reduce the loss of alloying elements, protecting it from saturation with gases.
When smelting in acid furnaces, after melting and removing the smelting slag, slag from broken glass (SiO2) is added. For final deoxidation, ferrosilicon, ferromanganese and aluminum are introduced into the ladle before releasing the metal.
In the main furnaces, deoxidation is carried out with a mixture of powdered lime, coke, ferrosilicon, ferromanganese and aluminum.
In the main furnaces, high-quality alloy steels with a high content of manganese, titanium, nickel, and aluminum are smelted, and in acid-lined furnaces, structural steels alloyed with other elements are smelted.
In furnaces it is possible to produce steels with low carbon content and carbon-free alloys, since there is no carburizing medium.
In vacuum induction melting, the inductor, crucible, batch dispenser and molds are placed in vacuum chambers. High-quality alloys with a low content of gases, non-metallic inclusions and alloys alloyed with any elements are obtained.
Watch the educational video "Equipment of an electric steel melting shop."