The use of pressure gauges for the safe operation of gas cylinders
Category: Technologies and solutions
Technical gases in different physical states are widely used in industry. For example, liquefied gas in cylinders is used for welding metal structures in bridge construction. This gas is also necessary for technological processes in medicine and metallurgy.
Cylinders with compressed gases, especially toxic and flammable ones, are a great danger. When working with them, extreme caution is required: if the pressure inside the cylinder goes beyond acceptable limits, for example, due to a fall or exposure to high temperature, the cylinder will explode. People and the environment may be harmed.
Organizations that use explosive gas cylinders in their activities:
manufacturers of gas cylinder monitoring systems
manufacturers of gas fire extinguishing systems
manufacturers of analytical equipment and chromatographs
A clear example of how a cylinder explodes and the danger it poses is shown in the video. It shows a fire at a welding materials warehouse in Dallas, Texas, USA.
One of the reasons for the explosion of cylinders is a violation of safety rules: labor protection, fire and industrial safety. Another is the use of pressure gauges that do not comply with the requirements of the “Rules for the design and safe operation of pressure vessels” (PB 03-576-03). Since gases can be partially corrosive, the materials from which the cylinder, pressure regulator and pressure gauge are made must also comply with these regulations.
Pressure gauges for monitoring residual pressure in gas cylinders
Where to buy a propane tank
Our company provides you with the opportunity to buy propane at a competitive price.
Our region has a wide network of certified companies selling and refilling cylinders. The most common are tanks with a capacity of 27 and 50 liters, but upon pre-order, you will be supplied with products of 5 or 12 liters in the shortest possible time.
When refueling, of course, it’s faster to exchange your cylinder for an already filled one. However, many people prefer to wait a little to get theirs back. After all, it definitely does not allow gas to pass through and passed the necessary tests on time. The operator himself ensures that the amount of liquid propane does not exceed 85% of the geometric capacity of the vessel. This is necessary so that when heated, the internal pressure does not exceed the permissible threshold. If there are residual contents at the bottom (so-called condensate), the refiller must drain it into a special container.
- What are the rules for storing and using gas cylinders?
- How to prepare metal for cutting? What determines the accuracy and quality of cutting?
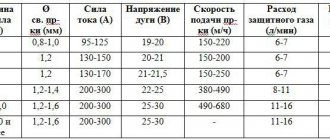
Cylinders are one of the most common power sources for gas-flame equipment, used to supply gas to individual working (welding) stations. Cylinders must comply with the requirements of the Rules for the Design and Safety of Operation of Pressure Vessels. Depending on the type of gas, they must be painted in different colors (table)
.
Acetylene cylinders.
Unlike all other compressed gases, acetylene is stored in seamless cylinders type 100 with a capacity of 40 liters, filled with a porous mass. Activated carbon BAU according to GOST 6217 or cast mass produced using special technology are used as a porous mass. The average amount of dissolved acetylene in a 40-liter cylinder is 5.5 m 3 or 6 kg. The maximum gas extraction from a cylinder with a porous mass is 1.0 m 3 / h, and with a cast mass - 1.5 m 3 / h.
The residual pressure in the cylinder supplied from the consumer for filling should not exceed 0.1 MPa (1 kgf/cm2) and should not be lower than 0.05 MPa (0.5 kgf/cm2).
Oxygen cylinders.
Compressed gaseous oxygen is stored and transported in hollow, seamless cylinders in accordance with GOST 949, type 150L, with a capacity of 40 dm 3. The maximum amount of oxygen in a cylinder of this type at the highest pressure is 6 m 3 or 8 kg. Oxygen cylinders must be degreased. The residual pressure in the cylinder must be at least 0.05–0.1 MPa (0.5–1.0 kgf/cm2).
Cylinders for propane-butane are made welded of three types in accordance with GOST 15860–84. Type 3 cylinders are mainly used for gas-flame processing.
The maximum operating pressure in cylinders is different for each liquefied gas. So, for propane the maximum operating pressure should not exceed 1.6 MPa (16 kgf/cm2), and for butane - 0.45 MPa (4.5 kgf/cm2).
Liquefied gases have a high coefficient of volumetric expansion, so the cylinders are filled in such a way that the vapor cushion in them is sufficient to absorb the liquid that expands when heated.
Cylinders for other compressible gases (hydrogen, nitrogen, urban, natural argon, etc.)
are manufactured seamlessly in accordance with GOST 949. For these gases, cylinders of type 150 and 150L are used, and for methane and compressed air, cylinders of type 200 or 200L are used.
Table.
Data on gas cylinders used in flame processing
| Dissolved | 2,5 (25) | White | Attached with a clamp | |
| Hydrogen | Compressed | 15 (150) | Dark green | |
| Flammable gas | – | 15 (150) | Red | Same |
| Oxygen | – | 15 (150) | Blue | 3/4 pipe right |
| Coke gas | – | 15 (150) | Red | Diameter 21.8 mm, 14 threads per 1″ left (thread profile according to GOST 6357) |
| Methane | – | 15 (150) | – | Same |
| Propane | Liquefied | 1,6 (16) | – | – |
| Natural gas | Compressed | 15 (150) | – | – |
| Shale gas | Same | 15 (150) | – | – |
Preparing the metal surface for cutting.
Before cutting, the surface of the metal being cut must be thoroughly cleaned of scale, rust, paint and dirt. For manual cutting, it is enough to clean a narrow strip (no more than 30–50 mm) with a cutter flame and clean it with a wire brush. Before mechanized cutting on stationary machines, sheets are usually straightened on sheet-straightening rollers and the entire surface is cleaned either chemically or mechanically (shot blasting).
Sheets must be laid horizontally on supports. The free space under the sheet should be equal to half the thickness of the metal being cut plus 100 mm.
Accuracy and quality of cutting.
The cutting accuracy and quality of the cut surface depend on the type of equipment used, cutting modes, requirements for the part, and its purpose.
According to their purpose, a distinction is made between oxygen cutting of workpieces with allowance for machining and cutting of parts, i.e. without subsequent processing.
Accuracy is determined by the maximum deviations of the dimensions of cut parts and workpieces from the nominal dimensions. Deviations occur due to displacement of the cutter axis as it moves along a given contour or due to deformation of the sheet under the influence of the thermal effect of the heating flame and internal stresses in the metal being cut. The cutting accuracy is also affected by the expansion of the cutting jet and the change in the angle of inclination of the cutter. Maximum deviations of cut parts and workpieces are set depending on their nominal dimensions and the thickness of the metal being cut. GOST 14792 provides three classes of accuracy for cutting metal with a thickness of 5 to 100 mm: for the first class, the maximum deviations are +1...±3 mm, for the second - +2...±4.5 mm and for the third - ±3.5...±5 .5 mm.
The quality of the cut surface for oxygen cutting is determined by a combination of indicators such as the deviation of the surface from the perpendicular and the roughness of the cut surface. For both indicators, three quality classes are established. The standard deviation of the surface from the perpendicular with a thickness of the metal being cut is 5–100 mm is 0.2–2.5 mm. Roughness is determined by the average depth of the grooves (irregularities) left by the cutting jet on the cut surface. The standard groove depth for the same thickness range is from 0.05 to 1 mm.
The depth of the grooves for a particular nozzle design depends on the pressure of the cutting oxygen, the speed of movement of the cutter and the type of fuel. The best quality of the cut surface with the smallest groove depth is obtained when cutting using liquid fuel. When cutting with natural gas, the cut surface is smoother than when cutting with oxygen-acetylene. There is no melting of the upper edge, the radius of curvature of which does not exceed 2 mm. The amount of melting is affected by the power of the preheating flame (at a given cutting speed) and the type of combustible gas.
training in labor protection, safe methods and techniques for performing work, first aid in case of accidents at work, introductory and initial briefing on labor protection in the workplace, on-the-job training and testing of knowledge of labor protection requirements, safe methods and techniques performance of work; preliminary and periodic medical examinations.
2. Before starting work, the employee must:
check and ensure the serviceability of the measuring instruments on gas cylinders
, equipment, fixtures and tools, fencing, ventilation;
check the stability of the cylinders
and the correctness of their fastening in the cells; make sure there are no flammable materials in the workplace.
3. An employee should not start work in case of the following violations of labor protection requirements: violation of the integrity of the gas cylinder
(presence of cracks or dents), as well as in the absence of a stamp on
the gas cylinder
with the date of its test;
malfunction of the gas reducer
(leakage of the union nut of the reducer, damage to the reducer housing, etc.); malfunction of the pressure gauge on the gearbox (absence of a stamp indicating annual testing or untimely completion of regular tests, broken glass or housing, immobility of the needle when gas is supplied to the gearbox, damage to the housing); insufficient lighting of the workplace and approaches to it; lack of exhaust ventilation when working in enclosed spaces; presence of explosive and fire hazardous materials in the work area; malfunction of tools, equipment, devices.
4. Gas cylinders
should be stored in one-story warehouses with light coatings, equipped with ventilation, without attics. Warehouse walls must be made of non-combustible materials; Windows and doors must open outwards. The height of the warehouse must be at least 3.25 m; lighting must be explosion-proof.
5. Floors in a warehouse must be made of materials that prevent sparking when metal objects hit them. Floors must be laid at least 0.1 m from ground level.
6. Acetylene, oxygen and liquefied gas cylinders
must be stored separately. The cylinders are installed in a vertical position with caps and plugs screwed on the valve fittings.
7. Cylinders
must be firmly secured with clamps or chains and protected from sunlight and exposure to heating devices and devices.
8. Gas cylinders
, installed indoors, should be located at a distance of at least 1 m from a heating radiator and at least 5 m from a heat source with an open fire.
9. When constructing a screen protecting cylinders
from heating, the distance between the cylinder and the heating device can be reduced to 0.5 m. The distance between
the cylinders
and the safety screen must be at least 10 cm.
10. When working in an open area on a sunny day, cover the cylinders with a piece of tarpaulin.
11. Cylinders
near the walls of buildings must be installed at a distance of at least 0.5 m from the doors and windows of the first floor and 3 m from the windows and doors of the basement and basement floors, as well as sewer wells and cesspools.
12. Placement of gas cylinders
at emergency (fire) exits from premises, from the main facades of buildings, in passageways with heavy traffic.
13. It is prohibited to store flammable materials and carry out work involving the use of open fire (forging, welding, soldering, etc.) within a radius of closer than 25 m from the cylinder warehouse.
14. It is prohibited to use gas cylinders
, the inspection period of which has expired, as well as in the presence of external damage (cracks, corrosion of the body, noticeable changes in shape, etc.), faulty valves, adapters.
15. Rejected cylinders must be marked “Reject”; The threads of such cylinders must be marked with notches to prevent further use.
16. It is prohibited to heat cylinders to increase pressure.
17. Transportation of gas-filled cylinders
must be carried out on spring vehicles or vehicles in a horizontal position with the obligatory installation of gaskets (wooden blocks, rubber or rope rings, etc.) between the cylinders.
18. Combined transportation of oxygen cylinders and cylinders with flammable gases
both filled and empty on all types of transport is prohibited, with the exception of the delivery of two cylinders on a special hand trolley to the workplace.
19. Cylinders must be moved on specially designed trolleys, containers and other devices that ensure a stable position of the cylinders. Carrying cylinders on arms or shoulders is not allowed.
20. Transportation of cylinders
indoors it is allowed to do it by tilting in a slightly inclined position.
21. It is necessary to securely strengthen the cylinders and install them in such a way that there is no possibility of impacts or objects falling on them from above, or contact with the oxygen cylinder, reducer and hoses of fats and oils.
22. Remove the cylinder cap
striking with a hammer, chisel or other tool that may cause a spark is prohibited. If the cap cannot be removed, the cylinder should be replaced.
23. When using cylinders, it is prohibited to remove the gas completely contained in them. The residual gas pressure in the cylinder must be at least 0.05 MPa (0.5 kgf/sq. cm).
24. When carrying out welding work, connecting an oxygen reducer
the cylinder should be accessed with a special key; Tightening the union nut of the gearbox with the cylinder valve open is prohibited.
25. During work, there should be no more than two cylinders at the welding station at the same time - with oxygen and flammable gas
.
26. If the pressure in the cylinders is higher than permissible, it is necessary to briefly open the valve to release part of the gas into the atmosphere or cool the cylinder with cold water in order to reduce the pressure. When releasing gas from a cylinder or purging a valve or burner, the worker must be on the side opposite to the direction of gas release.
27. Release of gases from cylinders
in a container with a lower operating pressure should be done through a reducer designed for this gas.
28. When performing work in winter, if the valve on the cylinder freezes, it should be heated only with hot water.
29. Work must be stopped: if the pressure in the vessel has risen above the permissible level; when detecting a malfunction of safety valves; if the pressure gauge is faulty; in the event of a fire that directly threatens a vessel under pressure.
30. Upon completion of work, it is necessary to: tidy up the workplace. Make sure that after work there are no smoldering objects left (rags, insulating material, etc.), and if there is smoldering, fill them with water; remove gas cylinders, hoses and other equipment to their designated places. In this case, you must make sure that the valves on the cylinders are closed and the gas is released from the hoses. Report any malfunctions noticed during work to your immediate supervisor.
Why control the residual pressure in the cylinder?
Another reason for a cylinder explosion is the mixing of gases. The pressure under which the gas is stored in the cylinder is 150-200 bar. As gas is consumed, the pressure decreases. If you do not control the residual pressure and allow it to drop below the required level, ambient air with moisture or gas will leak from an adjacent cylinder of one line. For example, gas welding involves two different gases: acetylene and oxygen. When properly proportioned, they produce an even burn when mixed. But when one gas is sucked into another, uncontrolled mixing occurs, which will cause an explosion.
Acetylene in cylinders
Acetylene is a colorless flammable gas C2H2 with an atomic mass of 26.04, slightly lighter than air. Has a pungent odor.
In industry, acetylene is usually obtained from calcium carbide (CaC2) by decomposing the latter with water.
Acetylene spontaneously ignites at a temperature of 335°C, a mixture of acetylene with oxygen ignites at a temperature of 297–306°C, and a mixture of acetylene with air ignites at a temperature of 305–470°C.
Acetylene is explosive under the following conditions:
with an increase in temperature over 450–500°C and pressure over 1.5–2 at (about 150–200 kPa); at atmospheric pressure, an acetylene-oxygen mixture containing acetylene from 2.3 to 93% explodes from a spark, flame, strong local heating, etc.;
under similar conditions, a mixture of acetylene with air explodes when the acetylene content in it is from 2.2 to 80.7%; As a result of prolonged contact of acetylene with silver or copper, explosive acetylene silver or copper is formed, which explodes when the temperature rises or when impacted.
An acetylene explosion can cause significant destruction and serious accidents: the explosion of 1 kg of acetylene releases approximately twice as much heat as the explosion of 1 kg of TNT and approximately 1.5 times more than the explosion of 1 kg of nitroglycerin.
Safety precautions when working with acetylene:
- The acetylene content in the air of the working area must be continuously monitored by automatic devices that signal if the permissible explosion-proof concentration of acetylene in the air is exceeded, equal to 0.46%;
- when working with acetylene cylinders, there should be no open flame or heating system nearby; It is prohibited to work with cylinders that are in a horizontal position, with loose cylinders, or with faulty cylinders; it is necessary to use non-sparking tools, lighting and electrical equipment only in explosion-proof versions;
- if an acetylene leak is detected from the cylinder (by smell and sound), it is necessary to close the cylinder valve as quickly as possible with a special non-sparking key;
- when heated, an acetylene cylinder can explode with extremely destructive consequences; in the event of a fire, it is necessary, if possible, to remove cold acetylene cylinders from the danger zone; the remaining cylinders should be constantly cooled with water or special compounds until they cool completely; when the acetylene coming out of the cylinder catches fire, it is necessary to quickly close the cylinder valve with a special non-sparking key and pour water over the cylinder until it cools completely; In case of a strong fire, fire extinguishing must be done from a safe distance; When extinguishing fires, it is recommended to use fire extinguishers containing a phlegmatizing concentration of nitrogen 70% by volume, carbon dioxide 57% by volume, water jets, sand, compressed nitrogen, asbestos sheet, electrically sprayed foam and water; When extinguishing a strong fire, fireproof suits, gas masks, etc. are used.
Use of acetylene in welding
Acetylene is the main flammable gas used in gas welding and is also widely used for gas cutting (oxy-fuel cutting). The temperature of the oxy-acetylene flame can reach 3300°C. Thanks to this, acetylene, compared to more accessible flammable gases (propane-butane, natural gas, etc.), provides higher welding quality and productivity.
The supply of acetylene stations for gas welding and cutting can be carried out from acetylene cylinders and from an acetylene generator.
To store acetylene, standard cylinders with a capacity of 40 liters, painted white, with the inscription “Acetylene” in red are usually used (PB 10-115-96, GOST 949-73). According to GOST 5457-75, technical dissolved acetylene grade B and gaseous are used for gas-flame processing of metals.
Dependence of acetylene pressure in the cylinder on ambient temperature (reference value):
| Ambient temperature, ºС | Acetylene pressure in the cylinder, kgf/cm2, no more |
| -5 | 13,4 |
| 0 | 14,0 |
| +5 | 15,0 |
| +10 | 16,5 |
| +15 | 18,0 |
| +20 | 19,0 |
| +25 | 21,5 |
| +30 | 23,5 |
| +35 | 26,0 |
| +40 | 30,0 |
Notes:
- The gas pressure in the cylinder must be measured by a verified pressure gauge of at least accuracy class 4 according to GOST 2425-88.
- The gas temperature is assumed to be equal to the ambient temperature at which the filled cylinder must be kept for at least 24 hours.
- The pressure in an acetylene cylinder is a reference value, because The amount of gas is determined only by weighing.
- The gas pressure in the cylinder at temperatures below - 5 ° C is not determined and is not standardized due to the complete dissolution of acetylene in acetone.
To accurately determine the presence of dissolved gases in the cylinder, it is necessary to weigh the cylinder with this gas. Merely measuring the pressure in a cylinder with dissolved gas is not a criterion for determining the presence of gas!
Sale and delivery of gas cylinders with acetylene
supplies enterprises (of various profiles) with technical gases: nitrogen, argon, acetylene, gas mixtures, technical oxygen, propane, and carbon dioxide. In addition to the supply of technical gases, the company specializes in the sale of gas cylinders produced in accordance with GOST 949-73 and GOST 15860-84 (for propane). Among the company's additional services, we can note services for repair, rental, purchase and re-examination of gas cylinders.
Use of pressure gauges on cylinders with hazardous gases
To accurately control the residual pressure in cylinders with oxygen, acetylene and other explosive gases and mixtures, specially designed pressure gauges are used, which engineers call “oxygen”. Such pressure gauges are distinguished by the fact that during the production process they undergo a special technological procedure for cleaning with dry air. From their internal parts that come into direct contact with oxygen, all oil and grease remaining after machining are removed. Otherwise, oxygen, coming into contact with fats or oils, will quickly oxidize them, the mixture will ignite and destroy the pressure gauge.
An example of a flammable pressure gauge installed on an oxygen line
Porous mass for filling acetylene cylinders
The porous mass is intended for filling acetylene cylinders. The porous mass includes a compacted filler based on granular charcoal and additionally contains “whiskers” of fiberglass material, predominantly basalt fiberglass. The technical result is increased reliability.
The present invention relates to the field of production, transportation and use of cylinder acetylene and can be used in the production of acetylene cylinders.
Acetylene is a soluble gas. Among the solvents, the most widely used in practice is acetone, poured into a container with a porous mass that provides a multiple increase in the active surface of the solvent. A very wide range of materials are used as a porous mass for filling acetylene cylinders (see Miller S. “Acetylene, its properties, production and use.” L., 1969), including fibrous (silk, viscose, leather, sponge, linen , animal hair, glass and mineral wool, asbestos), granular (diatomaceous earth, charcoal, pumice, silica gel, peat, bone meal, porous concrete, sawdust, brick, etc.), impregnated and monolithic masses. The main requirements for porous masses are chemical stability in contact with the steel of the cylinder, acetone and acetylene, high porosity and thermal conductivity, mechanical strength, gas absorption and low cost. Fibrous porous mass is known, used in the USA (see Welding J., 27, 1948, p. 445), consisting of an asbestos rope that tightly fills the internal cavity of the cylinder. The disadvantage of such a porous mass is low thermal conductivity, the active removal of asbestos dust with the acetylene gas flow and the harmful effects of asbestos on workers. A cast porous mass is known, used by Uraltekhgaz JSC (see TU 6-21-38-85 “Cylinders for soluble acetylene with cast porous mass"), containing quartz sand, calcium oxide hydrate and asbestos, which is a continuous porous block formed at elevated temperature and pressure as a result of a hydrothermal reaction between silicon oxide and calcium oxide hydrate. The disadvantage of such a porous mass is also the presence of asbestos, which causes the danger of pulmonary diseases in workers, both during the production process when filling cylinders, and during operation. A granular porous mass is known, widely used in Germany (English patent 834830, published in 1960), containing 65% charcoal (preferably beech or alder), 23% kieselguhr and 12% basic magnesium carbonate 4MgCO3Mg(OH)25H2O. Such a porous mass also has the above disadvantages inherent in coal-containing porous masses, namely the technological complexity of filling cylinders, which involves filling activated carbon through a narrow neck and then shaking it by freely lowering (impacting) the cylinder from a height of 0.7 mm onto a wooden base, and unstable density, causing constant shrinkage during operation, the need to tighten control over density indicators and more frequent repair replenishment of the cylinder. A porous mass is also known, developed and used by the Swedish company AGA /see. Swedish application 2266, NKI 26 B 44, appl. 03/25/1925 (USSR patent 3994, NKI 26 V 44, published 11/30/1927)/, “Porous mass for filling vessels intended for storing acetylene and other gases”), consisting of round or other shaped bodies from loose porous material filling the spaces between the pieces, while the bodies are made of fibrous, powdery or granular porous material, cemented with a binder and covered on the outside with a porous shell, stronger than the core, while the bodies are formed from kieselguhr and a binder with fibrous additives materials for strengthening, as well as additives of fibrous materials, are introduced into the shell of bodies or in the form of composite bodies containing an inner core of charcoal, covered with a shell of kieselguhr with a binder material, and loose kieselguhr is used to fill the gaps between these pieces. The disadvantage of such a porous mass is is a deficiency of kieselguhr, as well as the great technological complexity and labor intensity of preparing filler bodies and their low mechanical strength, causing shrinkage and the need for more frequent replenishment of the cylinder with charcoal. The porous mass MG-100 for acetylene cylinders was chosen as the closest prototype (see. auto St. USSR 39915, NKI 26 V 44; 17 d. 3; publ. 11/31/1934 “Porous mass for acetylene cylinders”), consisting of compacted granular filler based on activated charcoal with a grain size of 1 to 1.5 mm in diameter with a packed porosity of the mass of about 80% and a liter weight of about 300 g per 1 liter of internal volume of the cylinder. This prototype also has disadvantages of its analogues: low mechanical strength of the grains of the activated charcoal base, which causes active shrinkage during operation, and the need for more careful monitoring of the condition of the cylinder and more frequent repair replenishment of the cylinder with granular activated charcoal. The purpose of the present invention is to develop a porous mass for filling acetylene cylinders, devoid of the disadvantages of analogues and the prototype. This technical effect is achieved by the fact that the known porous mass containing a compacted filler based on granular wood activated carbon additionally contains “whiskers” of fiberglass material, for example, basalt glass fibers, which are randomly distributed in the volume of the filler and form a reinforcing frame that fastens a block of porous mass inside the cylinder. The authors are not aware of technical solutions with the features specified in the claims aimed at achieving the same goal as in the object claimed as an invention, therefore the proposed technical the solution meets the criterion of “significant differences”. The introduction of “whiskers” of fiberglass materials into the coal porous mass provides high mechanical stability against deformation from mechanical impact, eliminates settlement and changes in porosity along the sections of the acetylene cylinder during long-term operation. Thus, the proposed porous mass for filling acetylene cylinders provide the following advantages: - high chemical stability and non-toxicity of fibrous materials on a glass-like basis; — high stability of the geometric dimensions of the block placed inside the cylinder, compacted and fastened with piercing “whiskers” of fiberglass, and, as a consequence, stability of porosity during long-term operation; — high temperature stability and mechanical strength and, as a result, increased safety of operation of the acetylene cylinder; - low cost (large natural reserves of raw materials, high productivity of fiberglass production) and simplicity of technology for introducing fiberglass “whiskers” into the porous mass and into the shell of the cylinder. Based on the above, the proposed invention, in comparison with the prototype, ensures the achievement of a positive effect and has the criterion “positive effect” “The use of the proposed technical solution does not require additional re-equipment of enterprises. The first industrial testing of the proposed technical solution - “Porous mass for filling acetylene cylinders” will be carried out in 2000 at Lentekhgaz JSC.
Formula of invention
Porous mass for filling acetylene cylinders, including a compacted filler based on granular charcoal, characterized in that it additionally contains “whiskers” of fiberglass material, predominantly basalt fiberglass.
Solutions for safe handling of gas cylinders
To work with non-corrosive gas, pressure gauges with internal parts made of non-ferrous metal, stainless steel or Monel are required. Before making a decision, it is also worth checking with the manufacturer whether they test for helium leaks.
To monitor the residual pressure in the cylinder, we recommend using electrical contact pressure gauges. This choice will provide several advantages:
continuous gas supply
reduction in costs for additional cleaning of the cylinder when fully used
control of minimum and maximum pressure at the regulator outlet
Source
Acetylene
Acetylene
Technical acetylene C2H2 is produced in two types: dissolved, gaseous and must be manufactured in accordance with the requirements of GOST 5457-75* “Dissolved and gaseous technical acetylene. Technical conditions" according to technological regulations approved in the prescribed manner.
GOST 5457 applies to technical acetylene obtained from calcium carbide in stationary generators and it provides for the following grades of acetylene:
— dissolved acetylene grade A — volume fraction of acetylene not less than 99.5%;
— dissolved acetylene grade B, first grade — not less than 99.1%;
— dissolved acetylene grade B, second grade — not less than 98.8%;
— acetylene gas — not less than 98.5%.
Technical dissolved acetylene grade A is intended to power lighting installations, technical dissolved acetylene grade B and technical gaseous acetylene are intended for use as a combustible gas in the flame processing of metals. Acetylene impurities are allowed - air, hydrogen phosphorous, hydrogen sulfide, water vapor.
Dissolved acetylene is a solution of acetylene in acetone under pressure in a cylinder, evenly distributed in a porous mass. Acetylene gas is a colorless gas with a density of 1.173 kg/m3 at 0 °C and 101.3 kPa (760 mm Hg).
Acetylene is an explosive gas. With air it forms an explosive mixture, the lower flammability limit of which corresponds to the gas concentration in the mixture with air equal to 2.5% (by volume) at ( = 25° (GOST 12.1.0041). Since acetylene gas is lighter than air, then in case of leaks in In the upper points of poorly ventilated rooms, an acetylene-air mixture may form.
The auto-ignition temperature of acetylene is 335 °C. Mixtures of acetylene with oxygen or air with a very low acetylene content can explode at atmospheric pressure. Therefore, it is necessary to comply with the mandatory rules for the safe operation of gas equipment when welding with acetylene. Self-ignition of a mixture of pure acetylene and oxygen leaving the burner nozzle occurs at a temperature of 428 °C.
Acetylene is more often used than other combustibles for welding and cutting; it gives the highest flame temperature when combustion in oxygen (3100-3200 °C). Without compromising quality and performance, acetylene is replaced only for cutting with other flammable gases - propane, methane, kerosene vapor, etc. Technical acetylene is colorless, due to the impurities it contains, it has a strong unpleasant odor, is 1.1 times lighter than air, and dissolves in liquids. Being under a pressure of 0.15-0.20 MPa, it explodes from an electric spark or fire, as well as when rapidly heated above 200 ° C. At temperatures above 530 °C, explosive decomposition of acetylene occurs.
Safety requirements. In accordance with GOST 12.1.007-76, in terms of the degree of impact on a living organism, acetylene belongs to the 4th hazard class.
The acetylene content in the air of the working area must be monitored by automatic continuous devices that indicate when the permissible explosion-proof concentration of acetylene in the air is exceeded, as well as periodically using indicator tubes in accordance with GOST 12.1.014-84.
Acetylene production falls into category A for fire hazard, and class B-1 for explosive zones; B-1a; V-1b; V-1g.
Acetylene production premises must have supply and exhaust ventilation.
Compressed nitrogen, carbon dioxide fire extinguishers, asbestos sheets, and sand should be used as fire extinguishing agents.
In industry, acetylene is produced in three ways: the decomposition of calcium carbide (a compound of calcium with carbon - CaC2) with water, thermal-oxidative pyrolysis (decomposition) of heated natural gas with oxygen, and the decomposition of liquid hydrocarbons (oil, kerosene) with an electric arc. For welding and cutting, acetylene is obtained from calcium carbide.
Technical gaseous acetylene is transported through pipelines made of seamless steel pipes manufactured in accordance with GOST 8731-87 and GOST 8734-75. The acetylene pressure in the pipeline should be no more than 0.15 MPa (1.5 kgf/cm2), it is measured by a pressure gauge of accuracy class 2.5 according to GOST 2405-88, the dial of which should have the inscription “Acetylene”.
Pipeline painting is in accordance with GOST 14202-69.
Technical dissolved acetylene is filled into steel cylinders for dissolved acetylene with a porous mass (activated carbon or cast porous mass) and acetone.
In equipment, pipelines and devices operating in an acetylene environment, parts made of copper or copper alloys with a copper content of more than 65% should not be used.
Cylinders must be equipped with special types of VAB valves designed for acetylene cylinders.
VAB valves for acetylene cylinders are usually made of steel. The use of copper alloys containing more than 65% is unacceptable, since upon contact with acetylene, explosive acetylene copper appears.
This acetylene valve has a thread different from other types of valves, which eliminates the possibility of installing it on other cylinders.
The gas pressure in the cylinder must be measured with an acetylene pressure gauge of 4 MPa (40 kgf/cm) with an accuracy class of at least 4 according to GOST 2405-88. The temperature of the gas in the cylinder is taken equal to the ambient temperature in which the filled cylinder must be kept for at least 8 hours.
At a nominal pressure of 1.9 MPa (19.0 kgf/cm2) at 20 °C, the gas pressure in the cylinder in the temperature range from minus 5 to plus 40 °C must correspond to that indicated in the table:
Acetylene pressure in the cylinder depending on temperature
| Gas temperature, ºС | Gas pressure in the cylinder, MPa (kgf/cm2), no more |
| -5 | 1,34 (13,4) |
| 0 | 1,40 (14,0) |
| +5 | 1,50 (15,0) |
| +10 | 1,65 (16,5) |
| +15 | 1,80 (18,0) |
| +20 | 1,90 (19,0) |
| +25 | 2,15 (21,5) |
| +30 | 2,35 (23,5) |
| +35 | 2,60 (26,0) |
| +40 | 3,00 (30,0) |
The residual gas pressure in the cylinder is measured with an acetylene pressure gauge of 0.4 MPa (4 kgf/cm) of accuracy class 2.5 with a scale diameter of at least 100 mm according to GOST 2405-88.
Cylinders from the consumer must be supplied with a residual pressure corresponding to that indicated in the table:
Residual pressure of acetylene in the cylinder
| Gas temperature, ºС | Residual pressure in the cylinder, MPa (kgf/cm2), not less |
| Up to 0 | 0,05 (0,5) |
| From 0 to +15 | 0,10 (1,0) |
| +15 to +25 | 0,20 (2,0) |
| +25 to +35 | 0,30 (3,0) |
Acetone is added to the cylinders to the required level at dissolved acetylene manufacturing plants.
Acetone CH3COCH3. Technical acetone is used as an acetylene solvent in accordance with GOST 2768-84. “Technical acetone. Technical conditions".
According to the degree of impact on the body, acetone belongs to the 4th hazard class. Acetone vapors cause irritation and diseases of the upper respiratory tract. Acetone has a narcotic effect. With prolonged inhalation of vapors, acetone accumulates in the body and can be absorbed through intact skin. Acetone poisoning is possible when inhaling acetone vapor in a concentration exceeding the maximum permissible concentration. The maximum permissible concentration for acetone vapor in the air of the working area is 200 mg/m3.
Acetone grades:
• highest grade - mass fraction of acetone not less than 99.75%;
• 1st grade - no less than 99.50%;
• 2nd grade - no less than 99.00%.
Acetone impurities - water, methyl alcohol, acids. Acetone is a colorless, flammable liquid. Flash point - minus 18 °C; auto-ignition temperature 500 °C. Mixtures of acetone vapor with air are explosive. The flammability limits of acetone vapor in air: lower - 2.2; top - 13% (by volume). The density of acetone is 790 kg/m3. The vapor density of acetone is 2.6 kg/m3, the vapor density relative to air is 2.0.
When in contact with sodium peroxide or chromic anhydride, acetone ignites explosively.
All work with acetone should be carried out using supply and exhaust ventilation away from fire and sources of sparking.
Respiratory protection in emergency situations - gas mask grade A or BKF.
To extinguish an acetone fire, powder fire extinguishers, volumetric extinguishing agents, sand, an asbestos blanket, water and foam are used.
Acetone is used in acetylene cylinders as a solvent for acetylene to neutralize its explosive properties. Under normal conditions, the absorption capacity of acetone as an acetylene solvent is 25 volumes of acetylene per 1 volume of acetone. The solubility of acetylene in acetone decreases with increasing temperature. So, for example, at -20 °C 52 liters of acetylene are dissolved in 1 liter of acetone, and at +20 °C only 20 liters are dissolved.
Acetone is introduced into a cylinder with a porous mass at the rate of 225-230 g per 1 liter of cylinder capacity. Being in the pores of the mass, acetylene dissolved in acetone becomes explosion-proof and can be stored in a cylinder under pressure. The acetylene pressure in the filled cylinder should be no more than 19 kgf/cm2 according to the acetylene pressure gauge at a temperature of 20 °C.
How to determine how much technical gas is left in the cylinder?
You cannot completely consume liquefied gas from the cylinder. Empty containers are accepted for refilling with a residual pressure of at least 0.05 MPa, and in the case of acetylene - from 0.05 to 0.1 MPa. Such requirements make it possible to control the remaining gas and prevent the penetration of foreign substances from the environment into the vessel. If the contents of the container are completely used up, an additional washing operation must be performed. Empty cylinders are stored in a warehouse with safety caps or are refilled.
Storage of gas cylinders
The cylinders are intended for gas supply to individual (mobile) posts and for equipping gas discharge ramps. Cylinders for GPOM (gas-flame processing of metal) must comply with the requirements of the “Rules for the Design and Safety of Pressure Vessels”. They are painted in different colors depending on the type of gas. The name of the gas stored in it is written in paint on the cylinder.
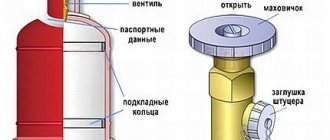
The upper spherical part of the cylinder is not painted; passport data is printed on it: cylinder type, cylinder serial number, manufacturer's brand, cylinder weight, cylinder capacity, operating and test pressure, date of manufacture and next test, quality control department and Rostekhnadzor stamp.
Oxygen cylinders . Compressed gaseous oxygen is stored and transported in hollow, seamless cylinders in accordance with GOST 949-73, type 150 and 150A (the numbers 150 indicate the pressure, and the letter “A” indicates that the cylinder is made of alloy steel) with a capacity of 40 liters.
The maximum amount of oxygen in a cylinder of this type at the highest pressure is 8 kg or 6 m3. The highest oxygen pressure in a cylinder is 15 MPa (150 kgf/cm2), and oxygen cylinders are tested at a pressure of 22.5 MPa (225 kgf/cm2). The outer diameter of the cylinder is 219 mm, wall thickness is 8 mm, length is 1390 mm, weight is 70 kg.
Cylinder color: blue.
The neck of the cylinder is equipped with a conical thread into which a brass valve is screwed. A plastic or metal cap is screwed onto the top of the cylinder neck to protect the valve from contamination and damage. At the bottom of the cylinder there is a shoe to give the cylinder vertical stability.
Oxygen cylinders must be degreased. It is always necessary to remember that oxygen cylinders and their fittings, including the reducer, must be protected from contamination with oil or fats, the slightest traces of which can spontaneously ignite in an oxygen environment and therefore pose a danger to the integrity of the cylinder. The residual pressure in the cylinder supplied from the consumer for filling must be at least 0.05-0.1 MPa (0.5-1.0 kgf/cm2).
Acetylene cylinders . Unlike other compressed gases, acetylene is stored in seamless cylinders type 100 with a capacity of 40 liters. The cylinders are filled with a porous mass soaked in acetone. Activated carbon BAU or cast mass made using special technology (infusor soil, crushed pumice and other porous materials) are used as a porous mass.
Acetone is used to dissolve compressed acetylene. Being in the smallest vapors of the mass and being dissolved in acetone, compressed acetylene loses its explosive properties and in this form can be completely safely stored under pressure up to 2.5 MPa (25 kgf/cm2). The average amount of dissolved acetylene is 5.5 m or 6 kg.
The maximum gas extraction from a cylinder with a porous mass is 1.0 m/hour, and from a cylinder with a cast mass—1.5 m/hour. The residual pressure in the cylinder supplied from the consumer for filling should not exceed 0.1 MPa (1 kgf/cm2) and should not be lower than 0.05 MPa (0.5 kgf/cm2).
The color of the cylinder is white.
The design of the valve for acetylene and oxygen cylinders is different, which eliminates the mistaken installation of an acetylene reducer on an oxygen cylinder and vice versa.
Cylinders for propane-butane . Three types of cylinders are manufactured in accordance with GOST 15860-84. For HPOM, type 3 cylinders are mainly used. The maximum operating pressure in cylinders for reduced gases is different for each of them. Thus, for propane the maximum operating pressure should not exceed 1.6 MPa (16 kgf/cm2), and for butane - 0.45 MPa (4.5 kgf/cm2).
The color of the cylinder is red.
Liquefied gases have a high coefficient of volumetric expansion, so the cylinders are filled in such a way that the vapor cushion in them is sufficient to absorb the liquid that expands when heated. The volume of gas in a 50-liter cylinder is about 11 m3.
Cylinders for other compressible gases (hydrogen, nitrogen, argon, urban, natural, etc.) are made seamless in accordance with GOST 949-73. For these gases, cylinders of type 150 and 150A are used, and for methane and compressed air, cylinders of type 200 or 200A are used.
Data on gas cylinders used in flame processing of metals
| Gas | State of the gas in the cylinder | Maximum operating pressure, MPa (kgf/cm2) | Cylinder color | Connection thread |
| Oxygen | Compressed | 15 (150) | Blue | 3/4″ pipe, right |
| Acetylene | Dissolved in acetone | 2,5 (25) | White | Attached with a clamp |
| Hydrogen | Dark green | |||
| Propane | Liquefied | 1,6-1,7 (16-17) | Red | |
| Argon grades I and II, technical | Compressed | 15 (150) | Black with white top | 3/4″ pipe, right |
| Helium | Brown | |||
| Carbon dioxide | Liquefied | 7,5 (75) | Black |
CYLINDER VALVES
Valve for acetylene cylinders . Designed for a working pressure of 2.5 MPa (25 kgf/cm2), it is made of steel and has a thread different from other valves. The balloon reducer is connected to the valve using a special O-shaped clamp, and opening and closing is done with a special socket wrench. Three types of acetylene valves are commercially produced, of which two (VBA and VAB) are with a membrane seal and one (VA) is with a gland seal.
Valve for oxygen cylinders . Designed for a working pressure of 20 MPa (200 kgf/cm2), made of brass. The VK-74 valve has a fluoroplastic seal in the valve, due to which the handwheel is rotated manually. All parts of oxygen valves must be thoroughly degreased and protected from contamination during operation. Oxygen cylinder valves can be used for nitrogen, helium, argon, carbon dioxide and compressed air.
Valve for propane-butane cylinders . Designed for a working pressure of 1.6 MPa (16 kgf/cm2). There are several models of propane-butane valves. They differ in the way they ensure tightness inside the gas cavity. For these purposes, membranes, rubber stockings, gaskets, etc. are used. All valves have a left-hand thread with a diameter of 21.8 mm (thread profile according to GOST 6357-81).
Weighing
The simplest method, accessible to every consumer and not requiring complex mathematical calculations. Before refilling a gas cylinder in Moscow or any other city, you need to study the container markings located on the bottom. The inscription must contain the manufacturer's data, the date of the last certification, the date of production, the nominal volume, dimensions, operating pressure and weight of the empty container. After this, all that remains is to weigh the cylinder with the remaining gas and determine the difference.
To roughly calculate the amount of content, you can multiply the resulting value by 2. This approximation is acceptable, since the weight of compressed household gas is approximately 0.5 kg/l. That is, if the difference in mass was 1 kg, then the amount of residue is about 2 liters. Knowing the remaining gas will help you calculate when to replace the cylinder.
Acetylene cylinders 40l
An acetylene cylinder with a capacity of 40 liters is intended for storing and transporting acetylene. The acetylene cylinder is equipped with an acetylene valve, a neck ring, a safety cap (new cylinders), and a support shoe. The cylinder is painted with white enamel paint.
On the top spherical part of the cylinder the passport data is stamped:
- manufacturer's trademark;
- cylinder number;
- date (month and year) of manufacture (test) and year of the next survey;
- test pressure P pr. MPa (kgf/cm2);
- working pressure P work. MPa (kgf/cm2);
- container weight (kg) - (weight of the cylinder without a cap, but with a porous mass and solvent, shoe, ring and valve);
- cylinder capacity in liters;
- date (month and year) of checking the condition of the porous mass;
- The quality control department stamp of the manufacturer is round, 10 mm in diameter.
For the safety of transportation and storage of acetylene under high pressure, the cylinders are filled with a cast porous mass. Cast porous mass - is a continuous porous block formed directly in the cylinder. A technological drilling with a diameter of 20 mm and a depth of 75 mm, filled with quartz grains, was made through the neck of the cylinder in the cast porous block. On the upper spherical part of cylinders manufactured before 1988, along with all passport data, the stamp of the porous mass filler plant “B21” is stamped, and on cylinders manufactured since 1988 the stamp “LM”. On cylinders with a cast porous mass, below the inscription “Acetylene” the letters “LM”, 60 mm high, are painted with paint. A metal mesh and a felt wad prevent the quartz grains and bulk porous mass from spilling out. To increase the gas collection capacity of the cylinders, the porous mass is impregnated with acetone in an amount of 9 to 13 kg. Acetylene cylinders in operation must be subject to periodic technical inspection, which includes:
- external inspection, which is carried out at each acceptance from the consumer, before checking the condition of the porous mass, testing for strength and density;
- checking the condition of the porous mass, which is carried out at least every 2 years;
- testing for strength and density under test pressure, P pr = 3.5 MPa (35 kgf/cm2), which is carried out at least once every 5 years on a pneumatic testing installation.
If the results are satisfactory, the date (month and year) of the test performed, the next test, and the stamp of the organization performing the certification are stamped on the cylinder. After checking the condition of the porous mass, the date (month and year) of the test performed and the stamp “PM” are stamped on the spherical part of the cylinder.
It is possible to place an order with RRR (Russian River Register) and RMRS (Russian Maritime Register of Shipping) certificates.
If you are interested, you can always contact us at the following address:
- Tel/fax: +7 (8443) 56-88-42
- Cell: 89044161316
Measurement with a special device
Modern manufacturers offer users equipment whose readings do not depend on external and internal factors. The measuring principle is based on ultrasound. The device is placed next to the cylinder and the degree of fullness is determined by the color indicator. By moving along the wall of the container from top to bottom, you can determine to what level it is still filled. The device is expensive, but it is very useful for the constant use of technical gases. Measurements are carried out quickly without additional manipulations with the cylinder and calculations.
Source
Acetylene.MAPP/MAF.Acetylene cylinders. Explosion hazard.
Acetylene cylinder Causes of cylinder explosions.
Acetylene cylinders (GOST 5948-51) are made from seamless pipes with a wall thickness of 7-8 mm. The weight of the shell of a 40 liter cylinder is on average 65 kg, and the weight of a charged cylinder is 82-85/st.
VNII Autogen has developed a design for a lightweight welded acetylene cylinder BAS-1-58. It is made of low-alloy steel 4 mm thick, with a water capacity of 60 liters. The weight of the equipped cylinder is 70-71 kg.
The acetylene pressure in the cylinder depending on the temperature is given below.
Temperature in ° C -10 -5 0 +5 +10 ZSH| +20 +25 +40
Pressure in atm. 7 8 9 10.5 12 14 16 18 25
During operation, cylinders are tested every five years with nitrogen at a pressure of 30 ati.
Acetylene cylinders are painted white and have the inscription “Acetylene” in red letters.
Inside, the acetylene cylinder is filled with a special highly porous mass impregnated with acetone, in which acetylene dissolves well. When storing acetylene in narrow channels of a porous mass, you can increase the pressure of acetylene in the cylinder to 15-16 atm without fear of its explosion. Dissolving acetylene in acetone is done in order to increase the amount of acetylene that can fit in the cylinder. Acetone is a liquid that dissolves acetylene well. One volume of acetone at one atmosphere pressure and room temperature dissolves 23 volumes of acetylene.
Birch activated carbon is used as a porous mass. The condition of the porous mass in the cylinder is checked by the filling plant annually.
When the valve of the cylinder is opened, acetylene is released from acetone in the form of a gas and flows through the reducer and hose into the burner. Acetone remains in the pores of the mass and dissolves new portions of acetylene during subsequent fillings. Acetone losses amount to 40-50 g per 1 m3 and occur due to the entrainment of acetone vapors along with acetylene gas. To reduce acetone losses, it is necessary to keep acetylene cylinders in a vertical position during operation.
When acetylene consumption exceeds 1500 l/h, several acetylene cylinders should be connected. Gas from the cylinder can be consumed to a residual pressure not lower than the following values:
Temperature in ° C……below 0° from 0 to +15° from +15 to + 25° from +25 to +35°
Residual pressure in kg/cm2 .0.5 1 2
At lower pressures, a significant vhoc of acetone with acetylene is observed.
To determine the amount of acetylene in a cylinder, you need to multiply the capacity of the cylinder in liters by the gas pressure in atmospheres and by a factor of 9.2, which takes into account the solubility of acetylene in acetone. For example, if the cylinder capacity is 40 liters, the acetylene pressure is 15 at, then the amount of acetylene in the cylinder will be equal to 40 X 15 X 9.2 = 5520 liters.
Valve device for acetylene cylinder
The acetylene cylinder valve is made of steel. The use of steel here is safe, but the use of copper and its alloys containing more than 70% copper is not allowed, since acetylene with copper can form explosive acetylene copper. The valve is opened and closed using a socket wrench placed on the square head of the spindle. The valve does not have a fitting. The gearbox is connected using a special clamp with a clamping bolt.
Rules for using cylinders. Transportation of cylinders over long distances should be carried out on spring vehicles. It is prohibited to transport oxygen and flammable gas cylinders together. During transportation, cylinders must be stacked with valves in one direction and supported by special wooden spacers with cutouts that prevent the cylinders from rolling over and hitting each other.
Cylinders with liquefied gases are transported in a vertical position with the valve facing up.
It is prohibited to load cylinders onto vehicles and trailers if there is dirt, debris and traces of oil in the body.
The combined transportation of filled and empty oxygen and acetylene cylinders on all types of transport is prohibited. It is allowed to transport two cylinders on a special hand trolley.
In summer, filled cylinders must be protected from heating by the sun's rays. The caps on the cylinders must be screwed in completely.
Loading and unloading of cylinders should be done carefully, avoiding impacts, shocks, and falls. Moving cylinders from one room to another should be done on special carts or stretchers, where the cylinder is tightly secured with a chain or clamp.
Moving cylinders from place to place within one room over a short distance is permitted by tilting.
Filled cylinders are stored in special rooms. If it is necessary to store cylinders outdoors, for example in the field, they must be protected from exposure to precipitation and sunlight by arranging wooden or tarpaulin canopies.
At the workplace, cylinders must be firmly secured in a vertical position to prevent them from falling, and there must also be canopies to prevent oil from falling on the cylinders (for example, from an overhead crane).
When performing installation work on construction sites, oxygen cylinders can be placed in a horizontal position on specially adapted stretchers. Cylinders must be located at a distance of at least 1 m from heating devices and at least 5 m from open flames.
Storing cylinders with gases - acetylene substitutes - at workplaces after work is completed is prohibited. Cylinders must be stored in a special storage room.
It is prohibited to remove the cap from the cylinder using a hammer, chisel or other means that can create a spark. If the cap does not unscrew, the cylinder must be sent to the filler plant (workshop).
When working indoors, you must carefully monitor the tightness of the cylinders.
If gas leakage is detected, the cylinder is removed to a safe place and, if it is impossible to close the valve, left under observation until the gas is completely released.
If a leak of flammable gases from a cylinder into a room is detected, work with open fire must be stopped immediately. Work can be resumed only after the cylinders have been removed and the room has been thoroughly ventilated.
If gas leakage is detected through the stuffing box, tightening the stuffing box nut should only be done with a wrench after closing the valve to the cylinder.
The use of a cylinder with a valve that allows gas to pass through is prohibited.
In cases where the gas cannot be used due to a malfunction of the cylinders, the cylinder must be sent to the filling plant (workshop) with the inscription in chalk “Caution—full.”
To open the acetylene cylinder valve, you must have a special socket wrench.
During operation, this key must be on the cylinder valve spindle at all times. The use of regular wrenches is prohibited.
If the oxygen cylinder valve freezes, heating should be done using clean hot water or steam. Usually the valve is heated by covering the upper spherical part of the cylinder and the valve itself with a rag soaked in hot water. In this case, it is necessary to ensure that the rags are not oily and that smoldering embers do not stick to them.
Do not heat the valve with a burner flame or heated metal.
In workshops with up to 10 welding stations, it is allowed to have no more than two oxygen cylinders and two flammable gas cylinders at each workplace. With a large number of posts, gas supply should be provided centrally from the ramp. Do not allow the cylinders and especially the shut-off valves to become contaminated with oil or grease. There should be fire extinguishers and sand boxes in the cylinder warehouse and work sites in case of fire. If a fire occurs, “cylinders must be immediately removed to a safe place (especially when they are filled).
Causes of cylinder explosions
Oxygen cylinders can explode for the following reasons:
1) when oil or grease gets into the cylinder or its fitting;
2) if there is any flammable gas in the oxygen cylinder (before filling with oxygen, the cylinder was used for flammable gas);
3) with too much gas extraction; in this case, the gas, passing at high speed through the valve, can electrify the neck of the cylinder and then a spark may occur. This phenomenon is especially often observed during the cutting process and when the cylinder is standing on a material that insulates it from the ground;
4) when the gas pressure in the cylinder is higher than permissible (the pressure may increase due to heating of the cylinder by sunlight or another heat source);
5) if the material is of poor quality, i.e., a decrease in thickness due to corrosion of the metal of the cylinder; When transported in winter, there may be a significant decrease in the ductility of steel, and then when the cylinder is struck, the metal may collapse.
6) when the valve and neck are dirty with calcium carbide.
When oxygen passes under the hood, an explosive mixture of oxygen and acetylene is formed.
Acetylene cylinders can explode for the following reasons:
1) with sharp shocks and impacts leading to the destruction of the metal of the cylinder or, as a rule, to the settling of the porous mass with the formation of voids in it. The settling of the mass, in turn, helps to increase the volume of the hollow space in the upper part of the cylinder. If the volume of the hollow space exceeds 75-150 cl3, then acetylene, released into this space and being in it under high pressure, becomes explosive;
2) with strong heating (over 30-40 ° C), which reduces the solubility of acetylene in acetone, as a result of which its pressure increases;
3) if the connection between the valve and the gearbox is not tight, as a result of which acetylene can escape into the atmosphere, creating the danger of an explosion of the acetylene-air mixture in the room and, as a consequence, of the acetylene cylinder.
https://electrowelder...-acetylene.html