Boiling point of propane at different pressures
One of the most important properties of propane and butane, which distinguishes them from other types of automotive fuel, is the formation of a two-phase liquid-vapor system at a free surface above the liquid phase, due to the appearance of saturated vapor pressure.
Rice. 1. Dependence of saturated vapor pressure of propane and butane on temperature
One of the most important properties of propane and butane, which distinguishes them from other types of automotive fuel, is the formation of a two-phase liquid-vapor system at a free surface above the liquid phase, due to the appearance of saturated vapor pressure, i.e. vapor pressure in the presence of the liquid phase in the cylinder.
During the process of filling the cylinder, the first portions of liquefied gas quickly evaporate and fill its entire volume, creating a certain pressure in it. When the pressure decreases, the gas instantly evaporates. The evaporation of liquefied gas in the cylinder continues until the resulting liquefied gas vapor reaches saturation.
This property of propane and butane allows gas to be stored in small volumes, which is very important. As an example, consider Fig. 1. The saturated vapor pressure of butane is 0.1 MPa at 0 °C and 0.17 MPa at 15 °C, and the saturated vapor pressure of propane at the same temperatures is 0.59 and 0.9 MPa, respectively.
This difference results in a significant difference in mixture pressure when the proportion of propane and butane changes. Pressure increases with increasing temperature, which leads to large changes in the volume of liquefied gas in a liquid state.
Therefore, if the liquefied gas in liquid state completely fills the cylinder and the temperature continues to increase, the pressure will increase rapidly, which may lead to the destruction of the cylinder.
Therefore, never fill the cylinder with liquefied gas completely. Be sure to leave a vapor cushion, the volume of which is equal to 10% of the full capacity of the cylinder.
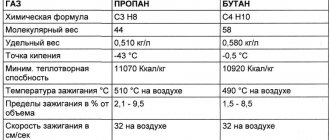
These two gases (propane and butane) differ in the boiling point at which they change from liquid to gaseous state. Propane stops turning into gas and remains in a liquid state at a temperature of -43 ° C; for butane this temperature is 0 ° C.
Rice. 2. Dependence of saturated vapor pressure of a mixture of propane and butane on temperature
In cold climates (or winter), liquefied petroleum gas—a mixture of propane and butane—intended for use as a vehicle fuel should have a predominance of propane for better gasification of the mixture.
Gas filling stations receive liquefied petroleum gas of two grades: summer GTBA - automotive propane-butane containing 50 + 10% propane, the rest is butane and winter PA - automotive propane containing 90 + 10% propane. The change in saturated vapor pressure P of a mixture of propane and butane depending on the temperature in the cylinder is shown in Fig. 2.
The heat of combustion of gas is slightly higher than that of gasoline. However, with an increase in the amount of air supplied to the engine, the heat of combustion decreases slightly.
If the power of an engine running on gasoline is taken as 100%, then the power of an engine running on gas will be approximately 90%, which leads to a decrease in maximum speed by about 4%, but do not forget about saving money. The world price ratio of gasoline to gas is 10:6.
The reduction in engine power occurs due to the lower heat of combustion of gas than that of gasoline (see Table 2). As a result, the engine cylinders are not completely filled with the gas-air mixture. Sometimes they try to eliminate this drawback by early setting the ignition timing to TDC by 3 - 5°. Under operating conditions, there is no big difference when driving a car on gas or gasoline.
Source
Chemical and physical properties
Propane-butane has unique chemical and physical properties, which literally makes it so popular among consumers all over the world.
Firstly, this representative of liquefied carbon gases remains in liquid form exclusively at high pressure, which is equal to 16 atmospheres. Therefore, during transportation, the substance is transported only in gas cylinders with appropriate pressure.
The combustion temperature of propane is not equal to any specific number and ranges between 800-1970 degrees Celsius. Such high indicators fully justify the benefits that it brings to human life, because the combustion of this mixture has high efficiency when performing any tasks related to the use of this gas.
The boiling point of propane is -42 degrees Celsius, which indicates a guarantee of safe operation under normal conditions.
But since we are considering a mixture of propane and butane, this figure can rise to -25 degrees and even higher, depending on the percentage of components in the substance. It is worth considering that propane freezes at a temperature of -188 degrees.
When transporting the substance, do not forget about the temperature of the propane in the cylinder, which should not exceed 15 degrees Celsius.
This approach is considered the safest, since when transporting gas cylinders at higher temperatures, the risk of fire increases significantly.
By the way, as for the ignition temperature of propane-butane, here they differ - for the first it is 504 degrees Celsius, and for the second - 430. But, despite such a large number of differences between its components, this representative of liquefied carbon gases is quite competitive with gasoline combustible mixtures.
Dependence of the boiling point of propane on pressure table. Liquefied petroleum gas
Liquefied
hydrocarbon gases
(propane-butane, hereinafter referred to as LPG) are mixtures of hydrocarbons that, under normal conditions (atmospheric pressure and air temperature = 0°C), are in a gaseous state, and with a slight increase in pressure (at a constant temperature) or a slight decrease in temperature (at atmospheric pressure) pass from a gaseous state to a liquid state.
Main components of LPG
are and .
Propane-butane (liquefied petroleum gas, LPG, in English - liquified petroleum gas, LPG) is a mixture of two gases. The composition of liquefied gas also includes in small quantities: propylene, butylene, ethane, ethylene, methane and liquid non-evaporating residue (pentane, hexane).
The raw materials for the production of LPG are mainly associated petroleum gases, gas condensate fields and gases obtained during oil refining.
From factories, LPG is supplied in railway tanks to gas filling stations (GFS) of gas facilities, where it is stored in special tanks until sold (dispensed) to consumers.
LPG is delivered to consumers in cylinders or by tanker trucks ().
In vessels (tanks, reservoirs, cylinders) for storage and transportation, LPG is simultaneously in 2 phases: liquid and vapor. LPG is stored and transported in liquid form under pressure created by the gas’s own vapors. This property makes LPG a convenient source of fuel supply for municipal and industrial consumers, because When stored and transported as a liquid, liquefied gas occupies hundreds of times less volume than gas in its natural (gaseous or vapor) state, and is distributed through gas pipelines and used (burned) in gaseous form.
Application area of LPG
Due to its environmental friendliness (clean combustion) and relatively low production and processing costs, propane-butane gas is widely used for the industrial and economic needs of the population. The scope of application of liquefied petroleum gas is wide. For example, LPG is used as a source of heat, fuel for vehicles, raw materials for the production of aerosols, as fuel for forklift trucks, etc.
- Industry In industry, liquefied hydrocarbon gases (propane-butane, isobutane) are used as raw materials and fuel. In the construction industry, SPBT (a mixture of propane and butane) is used in metal processing and gas welding work. The range of applications of LPG at large warehouse enterprises is wide. For example, SPBT is used for heating large warehouse and retail areas (in infrared heaters (emitters). Due to its environmental friendliness and odorlessness, gas is used as fuel for forklifts in grocery warehouses and in the food industry. LPG is widely used in the petrochemical industry. In The cosmetics industry uses isobutane in the production of sprays, auto chemicals.Isobutane is also used in the production of foamed insulating materials.
- Motor transport Propane-butane - liquefied hydrocarbon gas - is used as motor fuel as an alternative to the traditional type of fuel - gasoline. And it successfully competes with them on price. Today, with the advent of new advanced 4th generation LPG systems, converting vehicles to gas is becoming increasingly popular. A number of regional programs for converting cars to gas are currently being adopted. But due to the lack of proper funding, unfortunately, the process is being slowed down. Basically, the conversion of cars to gas occurs privately. But many motorists who use gas instead of gasoline note significant improvements in engine performance and real cost savings. Experts note the undeniable advantages of using liquefied petroleum gas instead of gasoline. For example, engine life increases by 10–15%, engine oil consumption decreases by 10%. When operating on gas, detonation does not occur at any engine operating mode. A gas-powered car has additional protection against theft and fuel drainage. A car owner who uses gas does not experience any inconvenience associated with refueling, because... Outwardly, the process of filling a car with gas is very similar to filling it with gasoline. And the number of gas stations is growing every day. Converting cars to gas is a real saving of your money.
- Utilities sector The traditional use of LPG is domestic use: for home heating with propane and cooking. Gas consumption volumes vary depending on the consumer: from small household plots to cottage villages and large construction projects. In private homes and enterprises where it is not possible to supply natural gas, it is advisable to use liquefied petroleum gas as fuel in boiler houses.
Technologies for obtaining LPG and LNG
To convert methane from gas to liquid, it must be cooled to -163 °C. And propane-butane liquefies at -40 °C. Accordingly, technologies and costs in both cases differ greatly.
One liter of LNG is equal to approximately 1.38 cubic meters. m of initial natural gas (this figure depends on temperature and pressure), decrease in volume - about 620 times
The following technologies from different companies are used to liquefy natural gas:
- AP-SMR (AP-X, AP-C3MR);
- Optimized Cascade;
- DMR;
- PRICO;
- MFC;
- GTL et al.
All of them are based on compression and/or heat exchange processes. The liquefaction operation takes place at the plant in several stages, during which the gas is gradually compressed and cooled to the temperature of transition into the liquid phase.
Preparation of the gas mixture
Before you can liquefy raw natural gas, you must remove water, helium, hydrogen, nitrogen, sulfur compounds and other impurities from it. For this purpose, adsorption technology is usually used for deep purification of the gas mixture by passing it through molecular sieves.
Then the second stage of feedstock preparation occurs, during which heavy hydrocarbons are removed. As a result, only ethane and methane (or propane and butane) with a volume of impurities of less than 5% remain in the gas, so that this fraction can begin to be cooled and liquefied.
Primary preparation with the removal of everything unnecessary from natural gas is carried out in order to protect refrigeration equipment from the aggressive effects of water, carbon dioxide, sulfur compounds, etc.
Fractionation allows you to get rid of harmful impurities and isolate only the main gas for subsequent liquefaction. At a pressure of 1 atm, the temperature of transition into the liquid state for methane is -163 °C, for ethane -88 °C, for propane -42 °C, and for butane -0.5 °C.
It is precisely these temperature differences that explain the reason why the gas entering the plant is divided into fractions and only then liquefied. There is no single liquefaction technology for all types of gaseous hydrocarbon compounds. For each of them it is necessary to build and use its own production line.
Basic liquefaction process
The basis for converting gas into a liquid state is the refrigeration cycle, during which heat is transferred by one or another refrigerant from an environment with a low temperature to an environment with a higher one. This process is multi-stage and requires powerful compressors for expansion/compression of the coolant and heat exchangers.
Compression technologies are high-tech, energy-intensive and costly, but in one cycle they allow gas to be compressed 5–12 times at once
The following are used as a refrigerant at different stages of liquefaction:
- propane;
- methane;
- ethane;
- nitrogen;
- water (sea and purified);
- air.
For example, for the primary cooling of natural gas at Novatek’s Yamal LNG, cool Arctic air is used, which allows the temperature of the feedstock to be lowered at minimal cost immediately to +10 °C. And in the hot summer months, it is instead provided for the use of sea water from the Arctic Ocean, which, regardless of the time of year, at a depth of constant 3–4 ° C.
At the same time, nitrogen, obtained directly on site from the air, is used as the final refrigerant in Yamal. As a result, the Arctic provides everything needed to produce LNG - from the initial natural gas to the working agents used in the liquefaction process.
Propane liquefies in a similar way to methane. Only it requires much lower cooling temperatures - minus 42 °C versus minus 163 °C. Therefore, liquefying gas for gas tanks costs several times less, but the resulting propane-butane LPG itself is less in demand on the market.
Transportation and storage
Almost the entire volume of LNG is transported by large-sized sea gas tankers from one coast to another. Transportation by land is limited by the need to maintain the temperature of the “liquid blue fuel” at values of about -160 ° C, otherwise methane begins to transform into a gas state and becomes explosive.
To transport LPG, cylinders of 5–50 liters with an internal pressure of up to 1.5–2 MPa and larger tank containers designed for 5–17 MPa are used.
The pressure in the LNG tank is close to atmospheric. However, if the temperature of liquid methane rises above -160 °C, it will begin to turn from a liquid into a gas. As a result, the pressure in the container will begin to increase, which poses a serious danger. Therefore, LNG tankers are equipped with low temperature maintenance units and a thick layer of heat insulation.
LPG is regasified into gas directly in the gas tank. And LNG regasification is carried out in special industrial installations without access to oxygen. According to physics, liquid methane at positive temperatures gradually turns into gas. However, if this happens directly in the air outside of special conditions, then such a process will lead to an explosion.
After natural gas in the form of LNG is liquefied at the plant, it is transported, and then again at the plant (regasification only) it is converted back into a gaseous state for further use.
From burning to recognition
Historically, the potential of gas as an energy source has been underestimated in our country. Not seeing economically justified areas of application, oil producers tried to get rid of light fractions of hydrocarbons and burned them uselessly. In 1946, the separation of the gas industry into an independent industry revolutionized the situation. The volume of production of this type of hydrocarbons has increased sharply, as has the ratio in Russia’s fuel balance.
When scientists and engineers learned to liquefy gases, it became possible to build gas-liquefaction enterprises and deliver blue fuel to remote areas not equipped with a gas pipeline, and use it in every home, as automobile fuel, in production, and also export it for hard currency.
What are liquefied petroleum gases
They are divided into two groups:
- Liquefied hydrocarbon gases (LPG) are a mixture of chemical compounds consisting mainly of hydrogen and carbon with different molecular structures, that is, a mixture of hydrocarbons of different molecular weights and different structures.
- Broad fractions of light hydrocarbons (NGL) - include mostly mixtures of light hydrocarbons of hexane (C6) and ethane (C2) fractions. Their typical composition: ethane 2-5%, liquefied gas fractions C4-C5 40-85%, hexane fraction C6 15-30%, the pentane fraction accounts for the remainder.
Liquefaction technologies
They learned to liquefy gases at the beginning of the 20th century: in 1913, the Nobel Prize was awarded to the Dutchman K. O. Heike for the liquefaction of helium. Some gases are brought to a liquid state by simple cooling without additional conditions. However, most hydrocarbon “industrial” gases (carbon dioxide, ethane, ammonia, butane, propane) are liquefied under pressure.
The production of liquefied gas is carried out at gas liquefaction plants located either near hydrocarbon fields or along the path of gas pipelines near large transport hubs. Liquefied (or compressed) natural gas can be easily transported by road, rail or water transport to the end user, where it can be stored, then converted back into a gaseous state and supplied to the gas supply network.
Special equipment
In order to liquefy gases, special installations are used. They significantly reduce the volume of blue fuel and increase energy density. With their help, it is possible to carry out various methods of processing hydrocarbons, depending on the subsequent application, the properties of the feedstock and environmental conditions.
Liquefaction and compression plants are designed for gas processing and have a block (modular) design or are completely containerized. Thanks to regasification stations, it becomes possible to provide even the most remote regions with cheap natural fuel. The regasification system also allows you to store natural gas and supply the required quantity depending on demand (for example, during periods of peak demand).
Most of the various gases in a liquefied state find practical application:
- Liquid chlorine is used to disinfect and bleach fabrics and is used as a chemical weapon.
- Oxygen - in medical institutions for patients with breathing problems.
- Nitrogen - in cryosurgery, for freezing organic tissues.
- Hydrogen is like jet fuel. Recently, cars powered by hydrogen engines have appeared.
- Argon is used in industry for metal cutting and plasma welding.
It is also possible to liquefy hydrocarbon gases, the most popular of which are propane and butane (n-butane, isobutane):
- Propane (C3H8) is a substance of organic origin of the class of alkanes. It is obtained from natural gas and by cracking petroleum products. A colorless, odorless gas, slightly soluble in water. Used as fuel, for the synthesis of polypropylene, the production of solvents, in the food industry (additive E944).
- Butane (C4H10), a class of alkanes. A colorless, odorless, flammable gas, easily liquefied. Obtained from gas condensate, petroleum gas (up to 12%), during cracking of petroleum products. Used as fuel in the chemical industry, in refrigerators as a refrigerant, in the food industry (additive E943).
How to properly heat a gas cylinder?
Now let’s look at how to ensure proper operation of gas equipment at low air temperatures, and what can be done to prevent gas from freezing. To resolve this issue, there are several options.
First of all, try to move the gas cylinder to a warm room; after some time, the frost from the surface will gradually evaporate, and inside the cylinder the conditions necessary for converting the liquefied gas into a vapor state will form. After this, the gas supply will be restored and the gas appliance can be used for its intended purpose.
But, if it is not possible to move the equipment, then it is necessary to heat the container on site so that the gas inside does not cool. Very often, owners of gas appliances resort to heating the cylinder by direct exposure to fire. It is strictly forbidden to perform such actions, as this contributes to the rapid transformation of gas into a vapor state, accordingly, the pressure in the container rapidly increases and can cause an explosion.
To reduce the likelihood of fuel cooling, you can insulate the cylinder with special materials that prevent the penetration of cold. But this method is suitable for small temperature changes in the environment.
In order to prevent freezing of a gas cylinder, you can insulate the container with a special material with a thermo-regulating base, but you cannot create the effect of a thermos
If the temperature outside is colder, then you can use special heating equipment. An electric heater can not only warm up a gas cylinder, but also provide a constant temperature at which the device will perform its functions with the greatest efficiency.
Thus, fuel consumption is reduced by up to 30 percent.
Characteristics of LPG
The main advantage of LPG is the possibility of their existence at ambient temperatures and moderate pressures in both liquid and gaseous states. In the liquid state they are easily processed, stored and transported; in the gaseous state they have better combustion characteristics.
The state of hydrocarbon systems is determined by the combination of influences of various factors, so for a complete characterization it is necessary to know all the parameters. The main ones that can be directly measured and affect flow regimes include: pressure, temperature, density, viscosity, concentration of components, phase relationships.
The system is in equilibrium if all parameters remain unchanged. In this state, no visible qualitative and quantitative metamorphoses occur in the system. A change in at least one parameter disrupts the equilibrium state of the system, causing one or another process.
Properties
When liquefied gases are stored and transported, their state of aggregation changes: part of the substance evaporates, transforming into a gaseous state, while part condenses and turns into a liquid. This property of liquefied gases is one of the determining ones in the design of storage and distribution systems. When boiling liquid is taken from reservoirs and transported through a pipeline, part of the liquid evaporates due to pressure loss, a two-phase flow is formed, the vapor pressure of which depends on the temperature of the flow, which is lower than the temperature in the reservoir. If the movement of a two-phase liquid through the pipeline stops, the pressure at all points is equalized and becomes equal to the vapor pressure.
Liquefied hydrocarbon gases
(propane-butane, hereinafter referred to as LPG) are mixtures of hydrocarbons that, under normal conditions (atmospheric pressure and air temperature = 0 ° C), are in a gaseous state, and with a slight increase in pressure (at a constant temperature) or a slight decrease in temperature (at atmospheric pressure) change from a gaseous state to a liquid state. The main components of LPG are propane and butane. Propane-butane (liquefied petroleum gas, LPG, in English - liquified petroleum gas, LPG) is a mixture of two gases. The composition of liquefied gas also includes in small quantities: propylene, butylene, ethane, ethylene, methane and liquid non-evaporating residue (pentane, hexane). The raw materials for the production of LPG are mainly associated petroleum gases, gas condensate fields and gases obtained during oil refining. From factories, LPG is supplied in railway tanks to gas filling stations (GFS) of gas facilities, where it is stored in special tanks until sold (dispensed) to consumers. LPG is delivered to consumers in cylinders or by tanker trucks. In vessels (tanks, reservoirs, cylinders) for storage and transportation, LPG is simultaneously in 2 phases: liquid and vapor. LPG is stored and transported in liquid form under pressure created by the gas’s own vapors. This property makes LPG a convenient source of fuel supply for municipal and industrial consumers, because When stored and transported as a liquid, liquefied gas occupies hundreds of times less volume than gas in its natural (gaseous or vapor) state, and is distributed through gas pipelines and used (burned) in gaseous form. Liquefied hydrocarbon gases supplied to populated areas must comply with the requirements of GOST 20448-90. For municipal consumption and industrial purposes, the standard provides for the production and sale of three brands of LPG: PT - technical propane; SPBT - technical mixture of propane and butane; BT - technical butane.
Why do they mix propane and butane in an autonomous gas supply system?
Considering the physicochemical characteristics of saturated hydrocarbons, their use largely depends on climatic conditions. Liquefied butane in its pure form will not work at subzero temperatures. Whereas the use of pure propane is contraindicated in hot climates, since high temperatures cause an excessive increase in pressure in the gas tank.
Since it is not practical to produce a separate grade of gas for each region, for the purpose of unification, GOST provides a mixture with a certain content of two components within the established standards. According to GOST 20448-90, the maximum butane content in this mixture should not exceed 60%, while for the northern regions and in the winter season the share of propane should be no less than 75%.
Percentage of gases at different times of the year
By the way, more articles from our blog about gasification are in this section.