Propane, production, properties, chemical reactions.
Propane, C3H8, is an organic substance of the alkanes class. It is found in nature in natural gas extracted from gas and gas condensate fields, and in associated petroleum gas. It is also formed during cracking of petroleum products.
Propane, formula, gas, characteristics
Physical properties of propane
Chemical properties of propane
Propane production
Chemical reactions - propane production equations
Applications and uses of propane
Application
Due to its properties, such as high calorific value during combustion, combustion without residue, harmlessness and safety when used correctly, ease of use, propane is a universal gas and is widely used both in production and in everyday life.
For industrial and domestic purposes it is supplied in the form of a technical propane-butane mixture. Butane (C4h20) is an organic compound of the alkanes class. Today, the demand for SPBT is huge. In production When performing gas-flame work in factories and enterprises: – in procurement production; – for cutting scrap metal; – for welding non-critical metal structures. During roofing work. For heating industrial premises in construction. For heating industrial premises (on farms, poultry farms, in greenhouses). For gas stoves, water heaters in the food industry. In everyday life - when preparing food at home and on the go; – for heating water; – for seasonal heating of remote premises – private houses, hotels, farms; – for welding pipes, greenhouses, garages and other utility structures using gas welding stations.
Recently, it has been widely used as automobile fuel, because... cheaper and more environmentally friendly than gasoline. In the chemical industry it is used to obtain monomers for the production of polypropylene. It is the starting material for the production of solvents. In the food industry, propane is registered as a food additive E944 as a propellant.
Refrigerant. A mixture of dried pure propane (R-290a) (a commercial designation to describe isobutane-propane mixtures) with isobutane (R-600a) is non-ozone depleting and has a low Greenhouse Potential (GWP). The mixture is suitable for functional replacement of obsolete refrigerants (R-12, R-22, R-134a) in traditional stationary refrigeration and air conditioning systems.
Quality indicators of liquefied hydrocarbon gases are determined according to GOST 10157-79.
Propane, formula, gas, characteristics:
Propane (lat. propanum) is an organic substance of the alkanes class, consisting of three carbon atoms and eight hydrogen atoms.
The chemical formula of propane is C3H8, the rational formula is CH3CH2CH3. Has no isomers.
Molecule structure:
Propane is a colorless gas, tasteless and odorless. However, odorants - substances that have a strong unpleasant odor - can be added to propane used as a technical gas to prevent its leakage.
It is found in nature in natural gas extracted from gas and gas condensate fields, and in associated petroleum gas. To separate natural and associated petroleum gas, they are purified and gas is separated.
It is also formed during cracking of petroleum products, incl. shale oil.
Also found in shale gas and LPG (liquefied natural gas).
Fire and explosion hazard.
Insoluble in water and other polar solvents. But it dissolves in some non-polar organic substances (methanol, acetone, benzene, carbon tetrachloride, diethyl ether and others).
Propane
According to toxicological characteristics, it belongs to substances of the 4th hazard class (low-hazard substances) according to GOST 12.1.007.
Propane. All aspects of use in everyday life and production of technical propane gas
The current level of technological progress allows people to use a variety of energy sources to solve their everyday problems. One of these resources is technical gas propane. This gas is familiar to absolutely all modern people. Propane is used almost everywhere today. This primarily concerns production processes. Thus, technical gas propane is successfully used for gas-flame work at various production facilities. It is used to perform both metal cutting and welding of non-critical structures. When working with scrap metal, this gas is practically indispensable for the procurement of raw materials. This is due to the fact that other technical gases for welding work are much more expensive and do not allow optimizing production costs.
Propane is also used with no less success in the production of thermal energy. Subsequently, the heat obtained using technical propane gas is used to provide heat supply to both industrial premises and to supply heat to residential complexes.
In everyday life, propane gas finds its use in a variety of areas of human activity. The most common way to use this gas is to use it as an energy carrier for gas stoves and geysers. With its help, a person cooks food and heats water. Also in the individual housing sector, propane is used to organize heating of premises. For this purpose, special equipment is installed. Propane gas is supplied to residential premises using gas pipelines. In some cases, delivery of liquefied propane in special cylinders may also take place.
Recently, propane has found another application in domestic conditions. It is used as automobile fuel. To do this, the vehicle is equipped with a set of equipment that allows the use of this technical gas to operate the engine. This type of optimization leads to significant savings for the motorist. The level of emissions of harmful substances from fuel combustion into the atmosphere is also significantly reduced.
It is worth knowing what propane gas is, what its advantages and disadvantages are. First of all, it is worth noting that technical propane gas is a synthetic product. It is often called natural gas. In part this statement is true. Indeed, propane is produced from natural gas, which is a residual product of the oil extraction process. This is the so-called accompanying gas.
In terms of its properties, propane gas is practically no different from natural gas. It has a high octane number. It also has excellent environmental and performance characteristics. Propane, which is supplied to consumers for use, is in a liquefied state. In order to ensure the liquefied state of gas, it is stored and transported under high pressure. However, you should know that if propane is supplied in cylinders, then the degree of filling of the container should not exceed 90%. This will ensure a high level of safety in the use of propane for domestic and industrial purposes.
Propane gas is not a toxic substance. It is safe enough for use if all technical operation requirements are met. Thus, propane cylinders should not be stored in residential areas. When storing them, ensure that they are not exposed to open heat sources or exposure to sunlight. Gas pipelines must be made of seamless steel pipes.
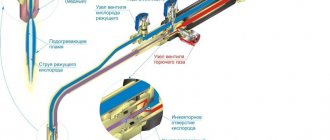
When performing welding work, technical gas propane is used in a mixture with oxygen. This gas successfully replaces acetylene in welding. At the same time, the undoubted advantage of propane is its lower cost and widespread availability. However, there are also disadvantages.
In particular, the combustion temperature of propane is almost a thousand degrees lower than the combustion temperature of acetylene. This creates certain difficulties during welding work. There are areas of work where the use of propane for gas-flame work can significantly reduce the quality of the work performed. Welding metal parts with a thickness greater than six millimeters using propane will be extremely difficult.
The main disadvantages of welding with propane include such side effects as a large percentage of part deformation, the presence of large grains in the weld itself and the heat-affected space. It is quite difficult to adjust the burner torch. In order to correctly set the reducing, oxidizing or normal burner mode for propane use, the welder must have extensive experience in such work.
For welding with technical propane gas, only injection gas torches are used. Today, almost all types of welding equipment torches and cutters offered on the market are injection-based. Therefore, the welder will not have any special problems with choosing suitable welding equipment for metal processing using propane.
The history of the use of propane in everyday life and production goes back an impressive period of time. Propane enters our lives more and more every year. This gas will be used for a long time. This is due to its cost-effectiveness, high consumer properties and accessibility to a wide range of consumers.
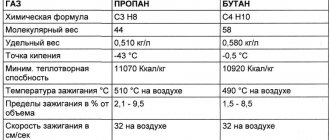
Physical properties of propane:
| Parameter name: | Meaning: |
| Color | no color |
| Smell | without smell |
| Taste | no taste |
| Physical state (at 20 °C and atmospheric pressure 1 atm.) | gas |
| Density (at 20 °C and atmospheric pressure 1 atm.), kg/m3 | 1,8641 |
| Density (at boiling point and atmospheric pressure 1 atm.), kg/m3 | 585 |
| Melting point , °C | -187,6 |
| Boiling point , °C | -42,09 |
| Auto-ignition temperature, °C | 472 |
| Critical temperature*, K | 370 |
| Critical pressure, MPa | 4,27 |
| Critical specific volume, m3/kg | 0,00444 |
| Explosive concentrations of gas-air mixture, % volume | from 1.7 to 10.9 |
| Specific heat of combustion , MJ/kg | 48 |
| Molar mass, g/mol | 44,1 |
* at temperatures above the critical temperature, the gas cannot be condensed at any pressure.
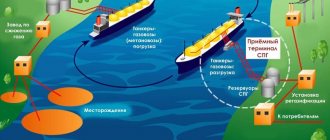
Two ways to obtain liquefied gas
Gas can be converted into liquid in two ways:
- by lowering the temperature;
- by compression under high pressure.
All gases condense into liquid naturally when its temperature drops below its boiling point. However, because these temperatures are often very low, requiring a lot of effort to create and maintain them, the high pressure method is more often used. When a gas is artificially compressed under high pressure, it can also take on a liquid form. However, this is not true for all substances.
Chemical properties of propane:
Propane is difficult to enter into chemical reactions. Under normal conditions, it does not react with concentrated acids, molten and concentrated alkalis, alkali metals, halogens (except fluorine), potassium permanganate and potassium dichromate in an acidic environment.
The chemical properties of propane are similar to those of other representatives of a number of alkanes. Therefore, it is characterized by the following chemical reactions:
- 1. catalytic dehydrogenation of propane:
CH3-CH2-CH3 → CH2=CH-CH3 + H2 (kat = Pt, Ni, Al2O3, Cr2O3, to = 575 °C).
- 2. propane halogenation:
CH3-CH2-CH3 + Br2 → CH3-CHBr-CH3 + HBr (hv or increased to);
CH3-CH2-CH3 + I2 → CH3-CHI-CH3 + HI (hv or increased to).
The reaction is chain in nature. A molecule of bromine or iodine under the influence of light breaks down into radicals, then they attack propane molecules, tearing off a hydrogen atom from them, as a result of which free propyl CH3-CH·-CH3 is formed, which collides with bromine (iodine) molecules, destroying them and forming new ones iodine or bromine radicals:
Br2 → Br++Br(hv); – initiation of the halogenation reaction;
CH3-CH2-CH3 + Br· → CH3-CH·-CH3 + HBr; – growth of the halogenation reaction chain;
CH3-CH·-CH3 + Br2 → CH3-CHBr-CH3 + Br·;
CH3-CH·-CH3 + Br· → CH3-CHBr-CH3; – break of the halogenation reaction chain.
Halogenation is one of the substitution reactions. The least hydrogenated carbon atom is halogenated first (tertiary atom, then secondary, primary atoms halogenate last). Propane halogenation occurs in stages - no more than one hydrogen atom is replaced in one stage.
CH3-CH2-CH3 + Br2 → CH3-CHBr-CH3 + HBr (hv or increased to);
CH3-CHBr-CH3 + Br2 → CH3-CBr2-CH3 + HBr (hv or increased to);
etc.
Halogenation will continue to occur until all hydrogen atoms have been replaced.
- 3. propane nitration:
See ethane nitration.
- 4. oxidation (combustion) of propane:
With excess oxygen:
C3H8 + 5O2 → 3CO2 + 4H2O.
Burns with a yellow flame.
When there is a lack of oxygen, instead of carbon dioxide (CO2), carbon monoxide (CO) is obtained, and with an even smaller amount of oxygen, finely dispersed carbon is released (in various forms, including in the form of graphene, fullerene, etc.) or a mixture of them.
- 5. propane sulfochlorination:
C3H8 + SO2 + Cl2 → C3H7-SO2Cl + … (hv).
- 6. propane sulfoxidation:
2C3H8 + 2SO2 + O2 → 2C3H7-SO2OH (increased to).
Advantages of liquefied gas
Converting gases into liquid form helped solve the problem of transporting and storing gases. Liquids are more convenient to store than gases because they do not require completely sealed rooms. The occupied volume is also significantly reduced: for methane it is reduced by 600 times.
Liquefied gas is easier to deliver and refuel, especially propane, butane, their mixture, and carbon dioxide. More information about how carbon dioxide cylinders are refilled can be found on this page of the TORGGAZ website.
However, methane is still used primarily in gaseous form due to the development of pipeline technology in the oil industry.
Getting propane. Chemical reactions - propane production equations:
Since propane is contained in sufficient quantities in natural gas, associated petroleum gas and is released during the cracking of petroleum products, it is not produced artificially. It is isolated during purification and separation from natural gas, APG and oil during distillation.
Propane in laboratory conditions is obtained as a result of the following chemical reactions:
- 1. hydrogenation of unsaturated hydrocarbons, for example, propene:
CH3-CH=CH2 + H2 → CH3-CH2-CH3 (kat = Ni, Pt or Pd, increased to).
- 2. reduction of haloalkanes:
C3H7I + HI → C3H8 + I2 (increased to);
C3H7Br + H2 → C3H8 + HBr.
- 3. interaction of haloalkanes with alkali metal, for example, sodium (Wurtz reaction):
C2H5Br + СH3Br + 2Na → CH3-CH2-CH3 + 2NaBr;
C2H5CI + CH3Cl + 2Na → CH3-CH2-CH3 + 2NaCl.
The essence of this reaction is that two haloalkane molecules bond into one, reacting with an alkali metal.
- 4. decarboxylation of butyric acid and its salts:
C3H7-COOH + NaOH → C3H8 + Na2CO3 (increased to);
C3H7-COONa + NaOH → C3H8 + NaHCO3.
Precautions for safe use
When liquefied propane comes into contact with air, it can form an explosive mixture. The maximum permissible value is considered to be the propane content in the air of the working area, not exceeding 300 mg/m³. It is unacceptable to have flame sources in rooms where there is even a slight odor of gas. Propane is heavier than air, and therefore it can settle and accumulate in areas with poor ventilation. Gas contact with an open area of skin is excluded - exposure to liquefied hydrocarbons causes frostbite, leading to tissue damage.
When working with propane, personal respiratory protection measures should be observed - inhaling propane has an intoxicating effect on the human body (dizziness, intoxication, loss of consciousness). Gas tends to accumulate in the body and is quickly eliminated, without accumulation in the internal organs.
Previous articleWhat you need to know about acetylene?
Next articleComposition and characteristics of welding mixtures
Propane-butane mixture
It has many advantages over other types of fuel, including natural gas:
- high efficiency;
- easy return to gaseous state;
- good evaporation and combustion at ambient temperature.
Propane fully meets these qualities, but butanes evaporate somewhat worse when the temperature drops to -40°C. Additives help correct this deficiency, the best of which is propane.
The propane-butane mixture is used for heating and cooking, gas welding and cutting of metals, as fuel for vehicles and for chemical synthesis.