Calculation of oxygen in cylinders
The parameters and dimensions of oxygen cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters.
According to GOST 5583-78 “Gaseous technical and medical oxygen” (Appendix 2), the volume of gaseous oxygen in a cylinder (V) in cubic meters under normal conditions is calculated by the formula:
Vb — cylinder capacity, dm3;
K1 - coefficient for determining the volume of oxygen in the cylinder under normal conditions, calculated by the formula
P - gas pressure in the cylinder, measured by a pressure gauge, kgf/cm2;
0.968 — coefficient for converting technical atmospheres (kgf/cm2) into physical ones;
t is the gas temperature in the cylinder, °C;
Z is the oxygen combustion coefficient at temperature t.
The values of the K1 coefficient are given in Table 4, GOST 5583-78.
Let's calculate the volume of oxygen in the most common cylinder in construction: a volume of 40 liters with a working pressure of 14.7 MPa (150 kgf/cm2). Coefficient K1 is determined according to Table 4, GOST 5583-78 at a temperature of 15°C:
V = 0.159 • 40 = 6.36m3
Conclusion (for the case under consideration): 1 oxygen cylinder = 40l = 6.36m3
Table 4. GOST 5583-78.
Gas temperature in the cylinder, °C
The value of the Ki coefficient at excess pressure, MPa (kgf/cm 2)
Properties of acetylene
In Soviet times, acetylene was used on construction sites by mixing calcium carbide with water. The unpleasant odor that accompanied the process was due to impurities of ammonia and hydrogen sulfide in the technical carbide. Pure acetylene itself:
- gas with a faint ethereal odor,
- slight sweetish taste,
- lighter than air
- slightly toxic.
The table below shows the basic values for the density, molar mass and weight of a liter of acetylene gas under normal conditions.
At natural humidity and normal temperature, acetylene is a colorless gas, and when the temperature drops to -85ºC it crystallizes.
A special characteristic that largely determined the areas of use of acetylene was its increased explosion hazard in a variety of accompanying conditions, for example, with a sharp increase in temperature (even as a result of friction) to 450 - 500ºC.
A mixture of air, and especially oxygen with acetylene, is explosive in a wide range of concentrations (from 2.8% to 81%). At 335ºС, gas spontaneously ignites. Compounds of acetylene with copper or silver explode on impact.
When the pressure on large volumes of gas increases above 2 kg/cm2, the gas becomes explosive and can detonate even from a spark of static electricity on clothing. The explosion is accompanied by an increase in pressure up to 10 times and temperature up to 3000 degrees Celsius.
To reduce explosiveness, acetylene is stored and transported in cylinders with fine-capillary porous filler or dissolved in acetone, nitrogen, methane, propane.
Calculation of propane-butane in cylinders
The parameters and dimensions of oxygen cylinders for propane, butane and their mixtures can be viewed according to GOST 15860-84. Currently, four types of these products are used, with volumes of 5, 12, 27 and 50 liters.
Under normal atmospheric conditions and a temperature of 15°C, the density of propane in the liquid state is 510 kg/m3, and butane 580 kg/m3. Propane in the gas state at atmospheric pressure and temperature 15°C is 1.9 kg/m3, and butane is 2.55 kg/m3. Under normal atmospheric conditions and a temperature of 15°C, 0.392 m3 of gas is formed from 1 kg of liquid butane, and 0.526 m3 from 1 kg of propane.
Let's calculate the weight of the propane-butane mixture in the most common cylinder in construction: volume 50 with a maximum gas pressure of 1.6 MPa. The proportion of propane according to GOST 15860-84 must be at least 60% (note 1 to Table 2):
50l = 50dm3 = 0.05m3;
0.05 m3 • (510 • 0.6 + 580 •0.4) = 26.9 kg
But due to the gas pressure limitation of 1.6 MPa on the walls, more than 21 kg cannot be filled into a cylinder of this type.
Let's calculate the volume of the propane-butane mixture in the gaseous state:
21kg • (0.526 • 0.6 + 0.392 •0.4) = 9.93m3
Conclusion (for the case under consideration): 1 cylinder = 50l = 21kg = 9.93m3
These gases are available from us: propane C3H8
Calculation of acetylene in cylinders
The parameters and dimensions of acetylene cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters. The body of an acetylene cylinder differs from the body of an oxygen cylinder in its smaller size.
At a pressure of 1.0 MPa and a temperature of 20 °C, a 40-liter cylinder holds 5–5.8 kg of acetylene by mass (4.6–5.3 m3 of gas at a temperature of 20 °C and 760 mm Hg).
The approximate amount of acetylene in the cylinder (determined by weighing) can be determined by the formula:
Va = 0.07 • E • (P – 0.1)
0.07 – coefficient, which takes into account the amount of acetone in the cylinder and the solubility of acetylene.
E – water volume of the cylinder in cubic dm;
P – pressure in the cylinder, MPa (pressure 1.9 MPa (19.0 kgf/cm2) at 20 °C according to GOST 5457-75 “Dissolved and gaseous technical acetylene”);
0.1 – atmospheric pressure in MPa;
Weight of 1 m3 of acetylene at a temperature of 0°C and 760 mmHg. is 1.17 kg.
Converting acetylene from m3 to kg
What do we want to learn today? How much does 1 cubic meter of acetylene, gas for welding, weigh, the weight of 1 m3 of acetylene?
No problem, you can find out the number of kilograms or the number of tons at once, the mass (weight of one cubic meter, weight of one cube, weight of one cubic meter, weight of 1 m3) is indicated in Table 1. If anyone is interested, you can skim the small text below and read some clarifications.
How is the amount of substance, material, liquid or gas we need measured? Except for those cases when it is possible to reduce the calculation of the required quantity to the counting of goods, products, elements in pieces (piece counting), it is easiest for us to determine the required quantity based on volume and weight (mass). In everyday life, the most common unit of volume measurement for us is 1 liter. However, the number of liters suitable for household calculations is not always an applicable way to determine the volume for business activities. In addition, liters in our country have not become a generally accepted “production” and trade unit for measuring volume. One cubic meter, or in its abbreviated version - one cube, turned out to be a fairly convenient and popular unit of volume for practical use. We are accustomed to measuring almost all substances, liquids, materials and even gases in cubic meters. It's really convenient. After all, their costs, prices, rates, consumption rates, tariffs, supply contracts are almost always tied to cubic meters (cubes), and much less often to liters. No less important for practical activities is knowledge of not only the volume, but also the weight (mass) of the substance occupying this volume: in this case we are talking about how much 1 cubic meter of acetylene gas weighs (1 cubic meter of welding gas mixture, 1 cubic meter of welding gas, 1 m3). Knowing mass and volume gives us a fairly complete idea of quantity. Site visitors, when asking how much 1 cubic meter of welding gas weighs, often indicate specific units of mass in which they would like to know the answer to the question. As we noticed, most often they want to know the weight of 1 cubic meter of acetylene gas (1 cubic meter, 1 cubic meter of welding gas mixture, 1 m3) in kilograms (kg) or tons (t). Essentially, you need kg/m3 or t/m3. These are closely related units that define quantity. In principle, a fairly simple independent conversion of weight (mass) from tons to kilograms and vice versa is possible: from kilograms to tons. However, as practice has shown, for most site visitors a more convenient option would be to immediately find out how many kilograms 1 cubic (1 m3) of acetylene weighs or how many tons 1 cubic (1 m3) of acetylene weighs
, without converting kilograms into tons or vice versa - the number of tons in kilograms per cubic meter (one cubic meter, one cube, one m3).
Therefore, in Table 1 we indicated how much 1 cubic meter of welding gas weighs (1 cubic meter of acetylene gas, 1 cubic meter) in kilograms (kg) and tons (t). Choose the table column that you need yourself. By the way, when we ask how much 1 cubic meter (1 m3) of acetylene weighs, we mean the number of kilograms of the gas welding mixture or the number of tons. However, from a physical point of view, we are interested in density or specific gravity. The mass of a unit volume or the amount of substance placed in a unit volume is the volumetric density of the gas welding mixture or the specific gravity of acetylene for gas welding. In this case, the volumetric density of the welding gas mixture and the specific gravity of acetylene.
The density of acetylene gas and the specific gravity of gas for welding in physics are usually measured not in kg/m3 or in tons/m3, but in grams per cubic centimeter: g/cm3. Therefore, in Table 1, specific gravity and density (synonyms) are indicated in grams per cubic centimeter (g/cm3)
Table 1. How much does 1 cube of acetylene, gas-welding mixture weigh, weight of 1 m3 of acetylene. Volume density of acetylene gas and specific gravity of acetylene for metal cutting in g/cm3. How many kilograms in a cube of gas for welding, tons in 1 cubic meter of welding gas mixture, kg in 1 cubic meter, tons in 1 m3.
Calculation of carbon dioxide (carbon dioxide) in cylinders
Carbon dioxide (according to GOST 8050-85 “Gaseous and liquid carbon dioxide”) is used as a shielding gas for electric welding work. Mixture composition: CO2; Ar + CO2 ; Ar + CO2 + O2. Manufacturers can also label it as a mixture of MIX1 - MIX5.
The parameters and dimensions of acetylene cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters.
At a working pressure of carbon dioxide in the cylinder of 14.7 MPa (150 kgf/cm2), filling factor: 0.60 kg/l; at 9.8 MPa (100 kgf/cm2) – 0.29 kg/l; at 12.25 MPa (125 kgf/cm2) – 0.47 kg/l.
The volumetric weight of carbon dioxide in the gaseous state is 1.98 kg/m³, under normal conditions.
Let's calculate the weight of carbon dioxide in the most common cylinder in construction: a volume of 40 liters with a working pressure of 14.7 MPa (150 kgf/cm2).
Let's calculate the volume of carbon dioxide in the gaseous state:
24kg / 1.98 kg/m3 = 12.12m3
Conclusion (for the case under consideration): 1 cylinder = 40l = 24kg = 12.12m3
Source
1a. SAFETY REQUIREMENTS
1a.1. Acetylene is an explosive gas. With air it forms an explosive mixture with a lower concentration limit of ignition at atmospheric pressure reduced to a temperature of 25°C - 2.5% (by volume) according to GOST 12.1.004-91.
The auto-ignition temperature of acetylene is 335°C.
1a.2. According to categories and explosion hazard groups, acetylene belongs to category and group IIC-T2 according to GOST 12.1.011-78*. ________________ * On the territory of the Russian Federation, GOST R 51330.2-99, GOST R 51330.5-99, GOST R 51330.11-99, GOST R 51330.19-99 are in force.
1a.Z. The acetylene content in the air of the working area must be monitored by automatic continuous devices that indicate when the permissible explosion-proof concentration of acetylene in the air is exceeded, as well as periodically using indicator tubes in accordance with GOST 12.1.014-84.
1a.4. Acetylene production falls into category A in terms of fire hazard, and in classes of explosive zones - classes B1, B1a, B1b, B1d.
1a.5. Acetylene production premises must have supply and exhaust ventilation.
1a.6. All work related to the production and use of acetylene must be carried out in accordance with the safety rules for acetylene production approved by the USSR State Mining and Technical Supervision.
1a.7. Compressed nitrogen, carbon dioxide fire extinguishers, asbestos sheets, and sand should be used as fire extinguishing agents.
Section 1a. (Introduced additionally, Amendment No. 2).
Specific gravity of acetylene. Weight of acetylene in 1m3
Acetylene gas was discovered back in 1836 by scientist Edmund Davy due to the action of water on calcium carbide. From 1855 to 1862, the French chemist Marcelin Berthelot was able to obtain acetylene, or as it was previously called “hydrogen bicarbonate,” in several ways, and he also gave it the name “acetylene.”
The bright, warm spectrum and hot flame of acetylene began to be used in lamps instead of gas lamps, not only at home, but also for street lighting and even as a lamp for bicycles and carriages.
ACCEPTANCE RULES
2.1. Dissolved and gaseous acetylene is taken in batches. A batch of dissolved acetylene is considered to be any quantity of acetylene that is homogeneous in its quality indicators, obtained in one technological cycle and accompanied by one quality document.
A batch of acetylene gas transported through a pipeline is any amount of acetylene sent to the consumer within 24 hours.
A batch of dissolved and gaseous acetylene sent to the consumer must be accompanied by a quality document containing the following data:
name of the manufacturer and its trademark;
name, brand and grade of product;
batch number;
date of manufacture of the product;
amount of acetylene in kilograms or cubic meters;
results of analyzes performed or confirmation of product compliance with the requirements of this standard;
designation of this standard.
(Changed edition, Amendment No. 2).
2.4. To check the quality of dissolved acetylene, the consumer selects 2% of the cylinders, but not less than two cylinders for a batch volume of less than 100 cylinders. The pressure in selected cylinders is checked.
2.5. If unsatisfactory analysis results are obtained for at least one of the indicators, a re-check should be carried out on a double sample or a double volume of samples from the same batch.
The results of repeated tests are final and apply to the entire batch.
ANALYSIS METHODS
3.1.1. To check the quality of acetylene, samples are taken from a pipeline or from a cylinder at 15-35°C using an acetylene reducer. Cylinders must be kept indoors for at least 8 hours.
3.1.2. Before determining the content of impurities, the first portion of acetylene must be released into the atmosphere, for which it is necessary to fully open the analysis valve on the pipeline or the valve on the cylinder for 2-3 s. The connecting tubes from the sampling point to the analysis installation must be purged with at least ten times the volume of acetylene to be analyzed. Acetylene is supplied to the installation under a pressure of no higher than 1000 mm water column.
Note. It is allowed to take acetylene samples into latex chambers for sports balls or oxygen pillows, having previously washed them 3-4 times with the analyzed gas. The residence time of the gas sample in the chamber and cushion should not exceed 30 minutes.
(Changed edition, Amendment No. 2).
3.1a. Determination of the volume fraction of acetylene
The volume fraction of acetylene ( ) in percent is calculated by the difference between 100 and the sum of the volume fractions of impurities according to the formula
where is the volume fraction of air and other gases that are poorly soluble in water, %;
— volume fraction of hydrogen phosphide, %;
— volume fraction of hydrogen sulfide, %;
— mass concentration of water vapor, g/m;
0.1335 is the conversion factor of g/m into the volume fraction of water vapor, expressed as a percentage.
(Introduced additionally, Amendment No. 2).
3.2. Determination of the volume fraction of air and other gases sparingly soluble in water
The volume fraction of air and other gases sparingly soluble in water is determined by a volumetric method based on the absorption of acetylene by running water and measuring the volume of unabsorbed gases.
(Changed edition, Amendment No. 1).
Damn.1. Installation for determining the content of air and other gases sparingly soluble in water in acetylene
Installation for determining the content of air and other gases sparingly soluble in water in acetylene
1 - bottle; 3-3 according to GOST 25336-82; 2, 5 - taps; 3, 7 — rubber tubes; 4 - burette; 6 - vessel
where is the mass of the empty burette, g;
Composition requirements
| Indicator name | Standard for dissolved acetylene | ||
| grade A | brand B | ||
| highest quality category | highest quality category | first quality category | |
| first class | second class | ||
| Volume fraction of acetylene, %, not less | 99,5 | 99,1 | 98,8 |
| Volume fraction of air and other gases sparingly soluble in water, %, no more | 0,5 | 0,8 | 1,0 |
| Volume fraction of hydrogen phosphide, %, no more | 0,005 | 0,02 | 0,05 |
| Volume fraction of hydrogen sulfide, %, no more | 0,002 | 0,005 | 0,05 |
| Mass concentration of water vapor at a temperature of 20 °C and a pressure of 101.3 kPa (760 mm Hg), g/m³, no more | 0,4 | 0,5 | 0,6 |
| which corresponds to the saturation temperature, °C, not higher | -26 | -24 | -22 |
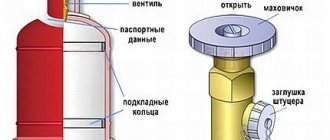
Technical dissolved acetylene is filled into steel cylinders for dissolved acetylene with a porous mass (activated carbon or cast porous mass) and acetone.
The cylinders are painted white and equipped with special types of valves designed for acetylene cylinders.
Transport markings must be applied to boxes with filled cylinders.
When sending filled cylinders by carload by rail, a label with transport markings must be attached to at least four cylinders at the doors of the car. For single shipments, a marking label is attached to each cylinder.
Transport markings may not be applied when transporting filled cylinders by road.
Dissolved acetylene in cylinders is transported by all modes of transport in accordance with the rules for the transportation of dangerous goods in force for this type of transport, and the rules for the design and safe operation of pressure vessels, approved by the State Technical Supervision Authority of the Russian Federation.
By rail, cylinders filled with dissolved acetylene are transported by carload and small shipments in covered wagons. When transporting in small shipments, the cylinder caps must be sealed.
To mechanize loading and unloading operations and consolidate transportation by road, medium-volume cylinders are placed in special metal containers.
Cylinders are transported by river transport in containers.
When transporting small-volume cylinders by all means of transport, they must be additionally packed in wooden lattice boxes of type VII in accordance with GOST 2991-76. Cylinders must be placed horizontally in boxes, with valves in one direction, with obligatory gaskets between the cylinders to protect them from hitting each other.
Boxes in the amount of two or more cargo pieces are subject to enlargement into transport packages in accordance with GOST 21929-76 with the main parameters and dimensions in accordance with GOST 24597-81 using fastening means in accordance with GOST 21650-76 and pallets in accordance with GOST 9078-84.
Source