Oxygen
Introduction
Oxygen is the most common element on earth, found in the form of chemical compounds in various substances: in the earth - up to 50% by weight;
in combination with hydrogen in water - about 86% by weight and in air - up to 21% by volume and 23% by weight. Under normal conditions (temperature 20 °C, pressure 0.1 MPa) it is a colorless, transparent, non-flammable gas, slightly heavier than air, odorless, but actively supporting combustion. Under normal conditions, the mass of 1 m3 of oxygen is 1.33 kg.
Oxygen has high chemical activity and is capable of forming chemical compounds (oxides) with all elements except inert gases (argon, krypton, xenon, neon and helium) and noble metals (gold, silver, platinum, palladium, rhodium, etc.) . The rate of the oxidation reaction increases sharply with increasing temperature or the use of catalysts. Oxidation reactions of organic substances in oxygen are exothermic in nature and proceed with the release of a large amount of heat. Increasing the pressure and temperature of oxygen in the reaction zone significantly accelerates it. In compressed or heated oxygen, the oxidation process under certain conditions can proceed at an increasing speed due to an increase in temperature in the reaction zone due to the release of heat.
Technical oxygen is widely used in many leading industries. It is used to intensify the smelting of steel (in open-hearth and electric furnaces) and cast iron (in blast furnaces), in oxygen-converter smelting of steel and in the production of non-ferrous metals from ores. A major consumer of oxygen is the chemical industry. With its use, gasification of solid fuels, conversion of gaseous hydrocarbons in the production of synthetic ammonia, methanol and formaldehyde, production of acetylene from natural gas, nitric and sulfuric acids and other processes are carried out.
Technical gaseous oxygen is used for gas-flame processing of metals and other technical purposes. Medical oxygen gas is used for breathing and medicinal purposes.
According to GOST 5583-78, oxygen differs in varying degrees of purity (99.7–99.2%). The importance of gas purity when welding and cutting metal should be taken into account. A decrease in oxygen purity by 1% not only degrades the quality of the weld, but also requires an increase in oxygen consumption by 1.5%.
Properties
The main properties of oxygen are given in Table 1.
Table 1
- Basic properties of oxygen
| Index | Indicator data |
| Formula | O2 |
| Molecular mass | 31,9988 |
| Density (at 0 °C and pressure 760 mm Hg), kg/m3 | 1,43 |
| Density (at 20 °C and pressure 760 mm Hg), kg/m3 | 1,33 |
| Critical temperature, °C | -118,8 |
| Critical pressure, kgf/cm2 | 51,35 |
| Boiling point (at 760 mm Hg), °C | -182,97 |
| Melting (solidification) temperature (at 760 mm Hg), °C | -218,4 |
| Mass of 1 liter of liquid oxygen at -182.97 °C and 760 mm Hg. Art., kg | 1,13 |
| Amount of gaseous oxygen obtained from 1 liter of liquid, l | 850 |
The mass concentration of mechanical impurities in medical oxygen intended for aviation is no more than 0.001 g/m3 with a particle size of no more than 0.1 mm at 15 °C and 101.3 kPa (760 mm Hg).
In terms of physical and chemical indicators, gaseous technical and medical oxygen must comply with the standards specified in Table 2.
Table 2
- Physico-chemical indicators of oxygen
| Indicator name | Standard for brands | ||
| Technical oxygen | Medical oxygen | ||
| First grade | Second grade | ||
| Volume fraction of oxygen, %, not less | 99,7 | 99,5 | 99,5 |
| Volume fraction of water vapor, %, no more | 0,007 | 0,009 | 0,009 |
| Volume fraction of hydrogen, %, no more | 0,3 | 0,5 | — |
| Volume fraction of carbon dioxide, %, no more | Not standardized | 0,01 | |
| Smell | Not standardized | Absence | |
Notes:
1. By agreement with the consumer, a volume fraction of oxygen in medical oxygen of at least 99.2% is allowed.
2. Medical oxygen intended for aviation must be produced with a volume fraction of water vapor of no more than 0.0007%.
3. In technical oxygen of the 2nd grade, produced in high-, medium- and two-pressure installations equipped with alkaline decarbonizers for purifying air from carbon dioxide, as well as in installations of the SKDS-70M type, a volume fraction of oxygen of at least 99.2% is allowed.
Production of oxygen from air
In industry, technically pure oxygen is produced in two ways:
- from the air - by deep cooling method;
- from water - by electrolysis.
The method of producing oxygen from air is more economical: 0.5–1.6 kW/h of electricity is consumed per 1 m3 of oxygen. To obtain 1 m3 of oxygen by electrolysis of water, 10–21 kW/h is required.
Atmospheric dehumidified air is a mixture containing 20.93% oxygen and 78.03% nitrogen, the rest is inert gases, carbon dioxide, etc. The content of water vapor in the air can vary depending on the temperature and the degree of saturation. To obtain technically pure oxygen, the air is deeply cooled and liquefied (the boiling point of liquid air at normal atmospheric pressure is –194.5 °C). The resulting liquid air is subjected to fractional distillation or rectification in distillation columns. The possibility of successful rectification is based on a fairly significant difference (about 13 °C) in the boiling temperatures of liquid nitrogen (–196 °C) and oxygen (–183 °C).
The air sucked in by a multi-stage compressor first passes through an air filter, where it is cleaned of dust, then passes through the compressor stages in succession. Behind each compressor stage, the air pressure increases and reaches 5–22 MPa, depending on the installation system and production stage. After each stage, the air passes through a water cooler and a moisture separator, where the water that condenses when the air is compressed is separated.
Compressed air from the compressor passes through a drying battery of cylinders filled with pieces of caustic soda, which absorbs moisture and remaining carbon dioxide. Then the compressed air enters the oxygen apparatus, where cooling, liquefaction and rectification (separation into oxygen and nitrogen) occur. Nitrogen gas is used as a shielding gas for copper welding.
Oxygen is sent to the gas holder and supplied to fill oxygen cylinders under pressure up to 16.5 MPa; the mass of 1 m3 of oxygen at normal atmospheric pressure (0.1 MPa) and 0 °C is 1.43 kg, at 20 °C – 1.31 kg; the mass of 1 liter of liquid oxygen is 1.13 kg; as a result of evaporation, 0.79 m3 of oxygen gas is formed (at 0 ° C and normal atmospheric pressure); 1 kg of liquid oxygen occupies a volume of 0.885 liters and, evaporating, forms 0.70 m3 of gaseous oxygen (at 0 °C and atmospheric pressure 0.1 MPa).
Calculation of the volume of oxygen gas in a cylinder
Volume of oxygen gas in the cylinder ( V
) in cubic meters under normal conditions is calculated using the formula:
| V = K 1· V b, |
Where
| V b | — | cylinder capacity, dm3. In the calculations, the average statistical value of the capacity of cylinders of at least 100 pieces is taken; |
| K 1 | — | coefficient for determining the volume of oxygen in a cylinder under normal conditions, calculated by the formula: |
Where
| P | — | gas pressure in the cylinder, measured by a pressure gauge, kgf/cm2; |
| 0,968 | — | coefficient for converting technical atmospheres (kgf/cm2) into physical ones; |
| t | — | gas temperature in the cylinder, °C; |
| Z | — | oxygen combustion coefficient at temperature t . |
K coefficient values
1 are shown in Table 3.
Table 3
— Coefficient values for determining the volume of oxygen in a cylinder
| Gas temperature in the cylinder, °C | Value of coefficient K1 at excess pressure, MPa (kgf/cm2) | ||||||||||||||
| 13,7 (140) | 14,2 (145) | 14,7 (150) | 15,2 (155) | 15,7 (160) | 16,2 (165) | 16,7 (170) | 17,2 (175) | 17,7 (180) | 18,1 (185) | 18,6 (190) | 19,1 (195) | 19,6 (200) | 20,1 (205) | 20,6 (210) | |
| -50 | 0,232 | 0,242 | 0,251 | 0,260 | 0,269 | 0,278 | 0,286 | 0,296 | 0,303 | 0,311 | 0,319 | 0,327 | 0,335 | 0,342 | 0,349 |
| -40 | 0,212 | 0,221 | 0,229 | 0,236 | 0,245 | 0,253 | 0,260 | 0,269 | 0,275 | 0,284 | 0,290 | 0,298 | 0,305 | 0,312 | 0,319 |
| -35 | 0,203 | 0,211 | 0,219 | 0,226 | 0,234 | 0,242 | 0,249 | 0,257 | 0,264 | 0,272 | 0,278 | 0,286 | 0,293 | 0,299 | 0,306 |
| -30 | 0,195 | 0,202 | 0,211 | 0,217 | 0,225 | 0,232 | 0,239 | 0,248 | 0,253 | 0,261 | 0,267 | 0,274 | 0,281 | 0,288 | 0,294 |
| -25 | 0,188 | 0,195 | 0,202 | 0,209 | 0,217 | 0,223 | 0,230 | 0,238 | 0,243 | 0,251 | 0,257 | 0,264 | 0,270 | 0,277 | 0,283 |
| -20 | 0,182 | 0,188 | 0,195 | 0,202 | 0,209 | 0,215 | 0,222 | 0,229 | 0,235 | 0,242 | 0,248 | 0,255 | 0,261 | 0,267 | 0,273 |
| -15 | 0,176 | 0,182 | 0,189 | 0,196 | 0,202 | 0,208 | 0,215 | 0,221 | 0,227 | 0,234 | 0,240 | 0,246 | 0,252 | 0,258 | 0,263 |
| -10 | 0,171 | 0,177 | 0,183 | 0,189 | 0,195 | 0,202 | 0,208 | 0,214 | 0,220 | 0,226 | 0,232 | 0,238 | 0,244 | 0,250 | 0,255 |
| -5 | 0,165 | 0,172 | 0,178 | 0,184 | 0,190 | 0,195 | 0,202 | 0,207 | 0,213 | 0,219 | 0,225 | 0,231 | 0,236 | 0,242 | 0,247 |
| 0 | 0,161 | 0,167 | 0,172 | 0,179 | 0,184 | 0,190 | 0,196 | 0,201 | 0,207 | 0,213 | 0,219 | 0,224 | 0,229 | 0,235 | 0,240 |
| +5 | 0,157 | 0,162 | 0,168 | 0,174 | 0,179 | 0,185 | 0,190 | 0,196 | 0,201 | 0,207 | 0,212 | 0,217 | 0,223 | 0,228 | 0,233 |
| +10 | 0,153 | 0,158 | 0,163 | 0,169 | 0,174 | 0,180 | 0,185 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,217 | 0,222 | 0,227 |
| +15 | 0,149 | 0,154 | 0,159 | 0,165 | 0,170 | 0,175 | 0,180 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,216 | 0,221 |
| +20 | 0,145 | 0,150 | 0,156 | 0,160 | 0,166 | 0,171 | 0,176 | 0,181 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,215 |
| +25 | 0,142 | 0,147 | 0,152 | 0,157 | 0,162 | 0,167 | 0,172 | 0,177 | 0,182 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,210 |
| +30 | 0,139 | 0,143 | 0,148 | 0,153 | 0,158 | 0,163 | 0,168 | 0,173 | 0,177 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 | 0,206 |
| +35 | 0,136 | 0,140 | 0,145 | 0,150 | 0,154 | 0,159 | 0,164 | 0,169 | 0,173 | 0,178 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 |
| +40 | 0,133 | 0,137 | 0,142 | 0,147 | 0,151 | 0,156 | 0,160 | 0,165 | 0,170 | 0,174 | 0,178 | 0,183 | 0,188 | 0,192 | 0,196 |
| +50 | 0,127 | 0,132 | 0,136 | 0,141 | 0,145 | 0,149 | 0,154 | 0,158 | 0,163 | 0,167 | 0,171 | 0,175 | 0,180 | 0,184 | 0,188 |
Transportation and storage
Packaging, labeling, transportation and storage of gaseous technical and medical oxygen - in accordance with GOST 26460.
The nominal oxygen pressure at 20 °C during filling, storage and transportation of cylinders and auto-recipients should be (14.7 ± 0.5) MPa [(150 ± 5) kgf/cm2] or (19.6 ± 1.0) MPa [ (200 ± 10) kgf/cm2].
Technical and medical oxygen is also transported by automobile gasification plants that gasify liquid oxygen directly at the consumer.
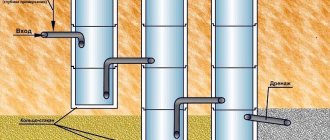
Technical oxygen is also transported through pipelines. The pressure of oxygen transported through the pipeline must be agreed upon between the manufacturer and the consumer. Oxygen is delivered to the welding site in oxygen cylinders, and in liquid form in special vessels with good thermal insulation.
To convert liquid oxygen into gas, gasifiers or pumps with liquid oxygen evaporators are used. At normal atmospheric pressure and a temperature of 20 ° C, 1 dm3 of liquid oxygen upon evaporation gives 860 dm3 of gaseous oxygen.
Return cylinders and auto-recipients must have a residual oxygen pressure of at least 0.05 MPa (0.5 kgf/cm2).
Safety requirements
Oxygen is not toxic, not flammable or explosive, however, being a strong oxidizing agent, it dramatically increases the ability of other materials to burn. Therefore, only materials approved for this purpose may be used for work in contact with oxygen.
When compressed gaseous oxygen comes into contact with organic substances, oils, fats, coal dust, flammable plastics, even in insignificant quantities, they may spontaneously ignite as a result of the release of heat during rapid compression of oxygen, friction and impact of solid particles on metal, as well as electrostatic spark discharge . Therefore, when using oxygen, care must be taken to ensure that it does not come into contact with flammable or combustible substances. Carbon steels can also ignite in oxygen if there is a sufficient amount of heat at the point of contact and a small mass of metal (for example, when thin plates rub against massive machine parts, the presence of chips, scale particles or iron powder).
To prevent accidents, all oxygen equipment, oxygen lines and cylinders are thoroughly degreased. It is necessary to exclude the possibility of ingress and accumulation of oils and fats on the surface of parts operating in an oxygen environment.
The cylinders of compressors that pump oxygen into cylinders are lubricated not with oil, but with distilled water, to which 10% glycerin is sometimes added. In addition, oxygen compressors use piston rings made of graphite and other antifriction materials that operate without lubrication and do not pollute oxygen with organic impurities.
Also dangerous are porous hot substances soaked in liquid oxygen (coal, soot, felt, tow, rags, cotton wool, etc.), which in this case become explosive. Clothes and hair, being saturated with oxygen, easily catch fire. Mixtures of oxygen with flammable gases, liquids and their vapors are explosive at certain ratios of oxygen and fuel in the mixture.
The accumulation of oxygen in indoor air creates the risk of fires. The volume fraction of oxygen in work areas should not exceed 23%. In rooms where an increase in the volume fraction of oxygen is possible, the presence of people should be limited and flammable materials should not be present. These rooms must be equipped with air control devices and exhaust ventilation for ventilation.
Before carrying out repair work or inspection of pipelines, cylinders, stationary and mobile recipients or other equipment used for storing and transporting gaseous oxygen, it is necessary to purge all internal volumes with air. It is allowed to begin work only after the volume fraction of oxygen in the internal volumes of the equipment has been reduced to 23%.
After being in an oxygen-enriched environment, smoking, using open flames and approaching fire is not allowed. Clothes should be aired for 30 minutes.
Cylinders, auto-recipients and pipelines intended for transporting technical and medical oxygen are prohibited from being used for storing and transporting other gases, and it is also prohibited to carry out any operations that may contaminate their internal surface and worsen the physical and chemical characteristics of the product.
When loading, unloading, transporting and storing cylinders, measures must be taken to prevent falling, hitting each other, damage and contamination of cylinders with oil. Cylinders must be protected from precipitation and heating by sunlight and other heat sources.