Metal corrosion - what is it: types and methods of combating
“Corrosion” is a term that we know as the process of spontaneous destruction of metal.
Every year, millions of tons of metal are “eaten up” by corrosion under the influence of physicochemical and chemical reactions that occur during interaction with the environment. The development of self-destruction can be either partial (local corrosion) or complete (complete corrosion), and it all depends on the duration and intensity of the destructive process. Corrosion is divided into chemical and electrochemical by type.
Chemical corrosion is caused by the interaction of the surface of metallic materials with a corrosive environment. This process of metal destruction occurs in liquids and gases, which, in turn, are not able to conduct electric current. It follows from this that chemical self-destruction is divided into gas corrosion, where destruction occurs precisely under the influence of gases at high temperatures, and corrosion in liquid non-electrolytes, which are of organic (oil, gasoline, kerosene, various alcohols, etc.) and inorganic origin (molten sulfur, liquid bromine, etc.).
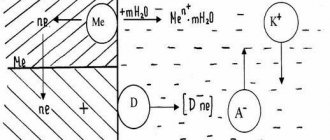
Electrochemical corrosion involves the destruction of metal in direct contact with an electrolytically conductive environment. This type of corrosion always requires the presence of an electrolyte with which the electrodes come into contact. It can also be two different metals with different redox properties that come into contact with each other and form a galvanic couple.
A galvanic couple is nothing more than a pair of conductors connected together for the purpose of making electrical contact, possibly made of different metals. Each metal has its own electrode potential. When exposed to an electrolyte, one will take on the role of the cathode, and the second the role of the anode, and a corrosion process will occur between them, as a result of which the cathode will destroy the anode. Moisture from the air is quite suitable as an electrolyte to activate the electrode potential of a galvanic pair, while the pairs are vulnerable to varying degrees: some more, others less.
Galvanic couple
In chemistry, there is a certain order of metals, where they are arranged in a sequence that characterizes their electrode potential in electrolyte solutions, and it is called the electrochemical series of metal voltages. This galvanic scale ( Diagram 1 ) can clearly help you understand why you should use fasteners made of a homogeneous material.
Scheme 1. Galvanic scale
So, based on this scale, we get the following: when two metals are in direct contact, the one to the left will corrode, and the one to the right will be more inertly protected. It is necessary to achieve a minimum potential difference between the two products:
- a difference of 0.1 will be acceptable safe;
- a difference of 0.2 will be acceptable if certain conditions are met: - contact corrosion will not affect the safety of the product and the loss of its working ability; — the assembly unit specifically provides for electrochemical protection of one product due to corrosion of another.
Also, the rate of corrosion will depend on the surface area of the exposed metals. Under conditions where the part is more inert than the fastener, the fastener will corrode at a more accelerated rate. For example, the use of galvanized hardware products to join stainless steels will lead to accelerated corrosion on the hardware and deterioration of their mechanical properties.
Galvanic couple
Protection against electrochemical corrosion; which metal will be the cathode and which anode in the galvanic pair; permissible, unacceptable and limitedly permissible metal contacts are regulated by GOST 9-005-72 “Electrochemical corrosion, permissibility of metal contacts”.
Table 1 provides reference data for some metals to determine compatibility.
Table 1. Reference data for some metals to determine compatibility
In this table you can see that the use of stainless steel and metal products coated with zinc is unacceptable and will lead to corrosion, which will reduce the service life of the products.
If it is not possible to exclude the formation of an unacceptable galvanic couple, it is worth taking additional steps to reduce contact corrosion using the following methods:
- additional installation of non-metallic washers, inserts or gaskets at joints;
- isolation of the connection from environmental influences;
- application of additional metal coatings that are compatible with each other;
- painting surfaces at joints;
- electrical insulation of metal products.
These procedures should be carried out based on the technical requirements for the product, the terms and conditions of their operation, and the economic component.
Neglecting the requirements for methods of protection against contact corrosion can lead to breakdown, loss of performance or destruction of products. It is possible that this will lead to additional material costs, moral or physical damage.
Corrosion of metals and alloys
Additional material for Chapter 8Metals and alloys are susceptible to destruction under the influence of the environment. The reason for this destruction lies in the chemical properties of metals - their ability to enter into redox reactions with environmental substances and oxidize.
The spontaneous destruction of metals and metallic materials (alloys) under the influence of the environment is called corrosion.
The term "corrosion" comes from the Latin word corrosio - "corrosion".
A well-known example of corrosion is the rusting of iron.
The amount of metal materials used in the national economy is very large. Many metals and their alloys (iron, aluminum, copper, lead, etc.) are susceptible to corrosion. Only noble metals are resistant to corrosion: silver, gold, platinum. Corrosion leads not only to the complete loss of metal products, but also to the loss of many valuable qualities of metals (hardness, ductility, etc.). Every year, due to corrosion, a huge amount of metal is irretrievably lost in the world, more than 20 million tons. Even more significant are the economic losses associated with damage to products due to corrosion, the costs of repairs, replacement of parts, equipment, devices, which are many times higher than the cost of the metals from which they are made. There are also many indirect losses due to metal corrosion: food spoilage, gas leaks, oil leaks from damaged pipelines, etc. Therefore, the fight against corrosion is the most important problem for humanity.
To fight corrosion, you need to know the essence of this process, the mechanism of its occurrence, the conditions that accelerate and slow down the destruction of the metal.
Problem.
What processes does corrosion belong to, what is its essence, what are the causes and conditions for the occurrence of this process?
There are different types of corrosion of metals and their alloys. The most common are two types: a) chemical corrosion and b) electrochemical.
Chemical corrosion is a type of corrosion caused by the direct interaction of a metal or alloy with dry gases, liquids that are not electrolytes, or solids. Its essence lies in the oxidation of the metal in the process of direct chemical interaction with environmental substances (gas, liquid corrosion).
An example of gas corrosion is the oxidation of iron in a chlorine atmosphere:
- 2Fe + 3Cl2 = 2FeCl3
or oxidation by atmospheric oxygen. When oxidized by air, films of a thin layer of oxides are formed on the surface of some metals, which subsequently protect these metals from corrosion. For example, such a film appears on the surface of nickel, chromium, copper, aluminum and other metals. To prevent corrosion, such a protective film is specially created on the surface of some metals, but it is denser, i.e. the metal is passivated (iron, chromium, titanium and other metals and their alloys).
Electrochemical corrosion is the most common type of corrosion, causing the greatest harm to metals and products made from them. Electrochemical corrosion occurs when two or more metals of the same alloy or metal come into contact with the surface of a product made of another metal in the presence of water or another electrolyte. In this case, a galvanic cell is formed, the electrodes of which are the metals in the electrolyte solution (water in which carbon dioxide is dissolved, acids, etc.). An electrochemical process occurs, i.e., along with chemical processes associated with the release of electrons and oxidation of the metal, electrical processes also occur (electron transfer from one section of the metal to another (Fig. 74).
When a galvanic couple occurs, the strength of the resulting electric current is greater, the farther the metals are from each other in the voltage series. In this case, the flow of electrons from the more active metal goes to the less active metal. The more active metal is destroyed. Thus, in the Fe-Zn pair, zinc is destroyed; in the Cu-Pt pair, copper corrodes.
Scheme of action of a galvanic couple (case of contact corrosion, a piece of copper on an iron surface, the environment is acidic):
Examples of electrochemical corrosion processes:
When iron comes into contact with tin, the opposite picture is observed: the intensity of iron corrosion increases.
End of paragraph >>>
Concept of metal corrosion
§ 5.
Most metals are easily destroyed under the influence of the external environment - air, water, solutions of acids, alkalis, salts, etc. The destruction of metals caused by the chemical or electrochemical influence of the external environment is called corrosion.
As a result of corrosion, all kinds of machines, mechanisms, instruments, and metal structures, which are more valuable in importance and cost than the metal used for their production, fail prematurely.
Based on the nature of metal destruction, corrosion can be divided into chemical and electrochemical.
Chemical corrosion occurs due to the reaction of a metal with dry gases and liquid dielectrics (i.e., liquids that do not conduct electrical current). An example of chemical corrosion is the oxidation of exhaust valves of internal combustion engines, flame tubes, furnace grates, etc.
The process of metal destruction during electrochemical corrosion is accompanied by the appearance of an electric current as a result of the interaction of the metal with its environment, which is an electrolyte. Electrochemical corrosion refers to the destruction of metal in solutions of acids, alkalis and salts. Alloys whose structure consists of crystals of different chemical compositions are easily susceptible to corrosion. Iron, aluminum, chromium and other metals, after exposure to oxidizing environments, become passive, i.e., they stop corroding. This is explained by the formation of a protective film of oxides on the surface of metals. Protective films are tarnish on the surface of steel and aluminum oxide (A1203) on the surface of aluminum.
Currently, the following methods have been developed to protect metals from corrosion:
alloying of alloys, which consists in introducing chromium, molybdenum, nickel into the steel alloy, which can prevent and reduce corrosion;
artificial creation of oxide films on the surface of metals or alloys (oxidation, anodization);
removal from the external environment of components that cause corrosion, for example, oxygen, which promotes corrosion, is removed from water, or about 0.5% chromium is introduced into it to reduce the activity of water, which eliminates corrosion in some cases;
application of metal protective coatings by galvanic, diffusion, spraying, cladding (rolling);
applying non-metallic coatings to the surface of metals (coating with a layer of varnishes and paints);
electrical protection (connecting a product to the negative pole of a current source), used to protect boilers and steel parts from corrosion during heat treatment in salt baths;
sacrificial protection, which consists in attaching a piece of metal or alloy (protector) active in a given environment to the structure being protected, which, when destroyed itself, protects the structure from corrosion.