Density of pure metals
| Name of material, brand | Density ρ, kg/m3 |
| Aluminum | 2700 |
| Beryllium | 1840 |
| Vanadium | 6500-7100 |
| Bismuth | 9800 |
| Tungsten | 19300 |
| Gallium | 5910 |
| Hafnium | 13090 |
| Germanium | 5330 |
| Gold | 19320 |
| Indium | 7360 |
| Iridium | 22400 |
| Cadmium | 8640 |
| Cobalt | 8900 |
| Silicon | 2550 |
| Lithium | 530 |
| Magnesium | 1740 |
| Copper | 8940 |
| Molybdenum | 10300 |
| Manganese | 7200-7400 |
| Sodium | 970 |
| Nickel | 8900 |
| Tin | 7300 |
| Palladium | 12000 |
| Platinum | 21200-21500 |
| Rhenium | 21000 |
| Rhodium | 12480 |
| Mercury | 13600 |
| Rubidium | 1520 |
| Ruthenium | 12450 |
| Lead | 11370 |
| Silver | 10500 |
| Waist | 11850 |
| Tantalum | 16600 |
| Tellurium | 6250 |
| Titanium | 4500 |
| Chromium | 7140 |
| Zinc | 7130 |
| Zirconium | 6530 |
Technical indicators of metal alloys
The most common copper-based alloys are brass and bronze . Their composition is also formed from other elements:
All alloys differ in structure. The presence of tin in the composition allows the production of bronze alloys of excellent quality. Cheaper alloys include nickel or zinc. The produced materials based on Cuprum have the following characteristics:
- high ductility and wear resistance;
- electrical conductivity;
- resistance to aggressive environments;
- low coefficient of friction.
Copper-based alloys are widely used in industrial production. They are used to produce dishes, jewelry, electrical wires and heating systems. Materials with Cuprum are often used to decorate the façade of houses and make compositions. High stability and ductility are the main qualities for the use of the material.
The density of a material is a physical quantity that determines the ratio of the mass of a material to its occupied volume. The unit of measurement for density in the SI system is kg/m3.
The values are averaged and are not reference values; the values of the indicated densities vary depending on the medium and measurement conditions.
One of the most common non-ferrous metals used in industry is copper, its Latin name is Cuprum, after the island of Cyprus, where it was mined by the Greeks many thousands of years ago. This is one of the seven metals that were known in ancient times; jewelry, dishes, money, and tools were made from it. Historians even called the period (from the 4th to the 3rd millennium BC) the Copper Age. D.I. Mendeleev put this metal in 29th place in his table, after hydrogen, since copper does not displace it from an acidic environment. Copper is a non-ferrous metal that has unique physical, mechanical, and chemical properties. The density of copper in kg m³ is one of the most important characteristics; it is used to determine the weight of the future product.
Density of ferrous metals
| Name of material, brand | Density ρ, kg/m3 |
| Steel 10 GOST 1050-88 | 7856 |
| Steel 20 GOST 1050-88 | 7859 |
| Steel 40 GOST 1050-88 | 7850 |
| Steel 60 GOST 1050-88 | 7800 |
| S235-S375 GOST 27772-88 | 7850 |
| St3ps GOST 380-2005 | 7850 |
| Malleable cast iron KCH 70-2 GOST 1215-79 | 7000 |
| High-strength cast iron HF35 GOST 7293-85 | 7200 |
| Gray cast iron SCh10 GOST 1412-85 | 6800 |
| Gray cast iron SCH20 GOST 1412-85 | 7100 |
| Gray cast iron SCh30 GOST 1412-85 | 7300 |
How is density determined?
The density of any substance is an indicator of the ratio of mass to total volume. The most common system for measuring density is kilogram per cubic meter. For copper this figure is 8.93 kg/m³. Since there are different grades of metal, which differ depending on the impurities of other substances, the overall density may vary. In this case, it is more appropriate to use another characteristic - specific gravity. In measuring systems, this indicator is expressed in different quantities:
Read also: Cutting wood with a band saw
Formula for determining the density of a substance
- SGS system - dyn/cm³;
- SI system - n/m³;
- MKSS system - kg/m³
In this case, the following formula can be used to convert values:
1 n/m³ = 1 dyne/cm³ = 0.102 kg/m³.
Specific gravity is an important indicator in the production of various materials containing copper, especially when it comes to its alloys. This is the ratio of the mass of copper to the total volume of the alloy.
You can consider how this indicator is used in practice using the example of calculating the weight of 25 copper sheets, 2000*1000 mm in size, 5 mm thick. First, let's determine the volume of the sheet - 5 mm * 2000 mm * 1000 mm = 10000000 mm3 or 10,000 cm³.
Specific gravity of copper 8.94 g/cm³
We calculate the weight of copper in one sheet - 10,000 * 8.94 = 89,400 g or 89.40 kg.
The mass of rolled copper in the total amount of material is 89.40 * 25 = 2,235 kg.
This calculation scheme is also used when processing scrap metal.
Density of stainless steels
| Name of material, brand | Density ρ, kg/m3 |
| 04Х18Н10 | 7900 |
| 08Х13 | 7700 |
| 08Х17Т | 7700 |
| 08Х20Н14С2 | 7700 |
| 08Х18Н10 | 7900 |
| 08Х18Н10Т | 7900 |
| 08Х18Н12Т | 7950 |
| 08Х17Н15М3Т | 8100 |
| 08Х22Н6Т | 7600 |
| 08Х18Н12Б | 7900 |
| 10Х17Н13М2Т | 8000 |
| 10Х23Н18 | 7950 |
| 12Х13 | 7700 |
| 12Х17 | 7700 |
| 12Х18Н10Т | 7900 |
| 12Х18Н12Т | 7900 |
| 12Х18Н9 | 7900 |
| 15Х25Т | 7600 |
Metal in nature
More than 6 thousand years ago, the first silver products began to be made in the Middle East. And when alloyed with gold, it served as the material for the world’s first coins. And for many centuries it has been valued by jewelers for its high quality, ease of processing and appearance.
Found in nature in nuggets. The largest nugget to date was found in Canada. Its length was 30 meters, and when melted down, 20 tons of metal were obtained. Unfortunately, the chemical activity of this metal allows it to be found only in the form of compounds, often they are silver salts, which contain selenium, sulfur, or other chemical elements. Metal reserves in the world today amount to about half a million tons, and the largest quantities are mined in Peru, China, Mexico, Australia and Chile.
Density of non-ferrous metal alloys
| Name of material, brand | Density ρ, kg/m3 |
| AL1 | 2750 |
| AL2 | 2650 |
| AL3 | 2700 |
| AL4 | 2650 |
| AL5 | 2680 |
| AL7 | 2800 |
| AL8 | 2550 |
| AL9 (AK7ch) | 2660 |
| AL11 (AK7TS9) | 2940 |
| AL13 (AMg5K) | 2600 |
| AL19 (AM5) | 2780 |
| AL21 | 2830 |
| AL22 (AMg11) | 2500 |
| AL24 (AC4Mg) | 2740 |
| AL25 | 2720 |
| B88 | 7350 |
| B83 | 7380 |
| B83S | 7400 |
| BN | 9500 |
| B16 | 9290 |
| BS6 | 10050 |
| BrAmts9-2L | 7600 |
| BrAZH9-4L | 7600 |
| BrAMZH10-4-4L | 7600 |
| BrS30 | 9400 |
| BrA5 | 8200 |
| BrA7 | 7800 |
| BrAmts9-2 | 7600 |
| BrAZH9-4 | 7600 |
| BrAZhMts10-3-1.5 | 7500 |
| BrAZHN10-4-4 | 7500 |
| BrB2 | 8200 |
| BrBNT1.7 | 8200 |
| BrBNT1.9 | 8200 |
| BrKMts3-1 | 8400 |
| BrKN1-3 | 8600 |
| BrMts5 | 8600 |
| BrOF8-0.3 | 8600 |
| BrOF7-0.2 | 8600 |
| BrOF6.5-0.4 | 8700 |
| BrOF6.5-0.15 | 8800 |
| BrOF4-0.25 | 8900 |
| BrOTs4-3 | 8800 |
| BrOTsS4-4-2.5 | 8900 |
| BrOTsS4-4-4 | 9100 |
| BrO3TS7S5N1 | 8840 |
| BrO3Ts12S5 | 8690 |
| BrO5TS5S5 | 8840 |
| BrO4Ts4S17 | 9000 |
| BrO4TS7S5 | 8700 |
| BrB2 | 8200 |
| BrBNT1.9 | 8200 |
| BrBNT1.7 | 8200 |
| LTs16K4 | 8300 |
| LTs14K3S3 | 8600 |
| LTs23A6Zh3Mts2 | 8500 |
| LC30A3 | 8500 |
| LTs38Mts2S2 | 8500 |
| LTs40S | 8500 |
| LS40d | 8500 |
| LTs37Mts2S2K | 8500 |
| LTs40Mts3ZH | 8500 |
| L96 | 8850 |
| L90 | 8780 |
| L85 | 8750 |
| L80 | 8660 |
| L70 | 8610 |
| L68 | 8600 |
| L63 | 8440 |
| L60 | 8400 |
| LA77-2 | 8600 |
| LAZ60-1-1 | 8200 |
| LAN59-3-2 | 8400 |
| LZhMts59-1-1 | 8500 |
| LN65-5 | 8600 |
| LMts58-2 | 8400 |
| LMtsA57-3-1 | 8100 |
| L60, L63 | 8400 |
| LS59-1 | 8450 |
| LZhS58-1-1 | 8450 |
| LS63-3, LMts58-2 | 8500 |
| LZhMts59-1-1 | 8500 |
| LAZ60-1-1 | 8200 |
| Ml3 | 1780 |
| ML4 | 1830 |
| Ml5 | 1810 |
| Ml6 | 1760 |
| Ml10 | 1780 |
| Ml11 | 1800 |
| Ml12 | 1810 |
| MA1 | 1760 |
| MA2 | 1780 |
| MA2-1 | 1790 |
| MA5 | 1820 |
| MA8 | 1780 |
| MA14 | 1800 |
| Kopel MNMts43-0.5 | 8900 |
| Constantan MNMts40-1.5 | 8900 |
| Cupronickel MnZhMts30-1-1 | 8900 |
| Alloy MNZh5-1 | 8700 |
| Cupronickel MH19 | 8900 |
| Alloy TB MN16 | 9020 |
| Nickel silver MNTs15-20 | 8700 |
| Kunial A MNA13-3 | 8500 |
| Kunial B MNA6-1.5 | 8700 |
| Manganin MNMts3-12 | 8400 |
| NK 0.2 | 8900 |
| NMTs2.5 | 8900 |
| NMTs5 | 8800 |
| Alumel NMtsAK2-2-1 | 8500 |
| Chromel T HX9.5 | 8700 |
| Monel NMZHMts28-2.5-1.5 | 8800 |
| TsAM 9-1.5L | 6200 |
| TsAM 9-1.5 | 6200 |
| TsAM 10-5L | 6300 |
| TsAM 10-5 | 6300 |
Properties and characteristics of metal
Iron is a fairly light, moderately refractory metal, silver-gray in color. Reacts easily with dilute acids and is therefore considered a medium activity element. In dry air, the metal is gradually covered with an oxide film, which prevents further reaction.
But at the slightest humidity, instead of a film, rust appears - loose and heterogeneous in composition. Rust does not prevent further corrosion of iron. However, the physical properties of the metal, and, most importantly, its alloys with carbon, are such that, despite the low corrosion resistance, the use of iron is more than justified.
Next, you will find out what the density of iron is (in kg per m3) in comparison, for example, with copper or aluminum.
Mass and density
The molecular weight of iron is 55.8, which indicates the relative lightness of the substance. What is the density of iron? This indicator is determined by the phase modification:
- α-Fe – 7.87 g/cubic. cm at 20 C, and 7.67 g/cc. cm at 600 C;
- The γ-phase has an even lower density - 7.59 g/cc at 1000C;
- The density of the δ-phase is 7.409 g/cc.
With increasing temperature, the density of iron naturally decreases.
Now let's find out what the melting point of iron is in Celsius, comparing it, for example, with copper or cast iron.
Temperature Range
The metal is moderately refractory, which means a relatively low temperature of change in the state of aggregation:
- melting point – 1539 C;
- boiling point – 2862 C;
- Curie temperature, that is, loss of the ability to magnetize, is 719 C.
It is worth keeping in mind that when they talk about the melting or boiling point, they are dealing with the δ-phase of the substance.
This video will tell you about the physical and chemical properties of iron:
Mechanical characteristics
Iron and its alloys are so widespread that, although they began to be used later than, for example, copper and bronze, they have become unique standards. When comparing metals, they point to iron: stronger than steel, 2 times softer than iron, and so on.
The characteristics are given for a metal containing small proportions of impurities:
- hardness on the Mohs scale – 4–5;
- Brinell hardness – 350–450 MN/sq. m. Moreover, chemically pure iron has a higher hardness – 588–686;
Strength indicators are extremely dependent on the amount and nature of impurities. This value is regulated by GOST for each grade of alloy or pure metal. Thus, the compressive strength for unalloyed steel is 400–550 MPa. When hardening this grade, the tensile strength increases to 700 MPa.
- the impact strength of the metal is 300 MN/sq m;
- yield strength –100 MN/sq. m.
We will learn further about what is needed to determine the specific heat capacity of iron.
Heat capacity and thermal conductivity
Like any metal, iron conducts heat, although its performance in this area is low: in terms of thermal conductivity, the metal is inferior to aluminum - 2 times less, and copper - 5 times.
Thermal conductivity at 25 C is 74.04 W/(m K). The value depends on temperature;
- at 100 k the thermal conductivity is 132 [W/(m.K)];
- at 300 K – 80.3 [W/(m.K)];
- at 400 – 69.4 [W/(m.K)];
- and at 1500 – 31.8 [W/(m.K)].
Important:
- The coefficient of thermal expansion at 20 C is 11.7·10-6.
- The heat capacity of a metal is determined by its phase structure and depends rather complexly on temperature. With an increase to 250 C, the heat capacity slowly increases, then increases sharply until the Curie point is reached, and then begins to decrease.
- The specific heat capacity in the temperature range from 0 to 1000C is 640.57 J/(kg K).
Electrical conductivity
Iron conducts current, but not nearly as well as copper and silver. The electrical resistivity of the metal under normal conditions is 9.7·10-8 ohm·m.
Since iron is ferromagnetic, its performance in this area is more significant:
- saturation magnetic induction is 2.18 Tesla;
- magnetic permeability – 1.45.106.
The toxicity of iron is discussed next.
Density of the rare metal osmium
It is found in small quantities on our planet. Most often it is found in the form of alloys with iridium and platinum, as well as in the form of oxides. Osmium has an HCP lattice with parameters a = 2.7343 and c = 4.32 angstroms. The average mass of one atom is m = 190.23 amu.
The above figures are sufficient to determine the value of ρ. To do this, you should use the original formula for density and take into account that one hexagonal prism contains six atoms. As a result, we arrive at the working formula:
Substituting the numbers written above and taking into account their dimensions, we come to the result: ρ = 22,579 kg/m 3.
Thus, the density of the rare metal is 22.58 g/cm 3, which is equal to the experimentally measured tabular value.
Who knows the metal with the lowest density?
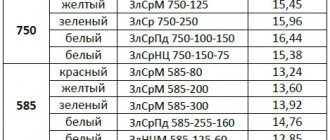
POS 61 tin 64%, lead 36% 181 T, P sodium 70%, mercury 30% 181 T Chem. act, Toxic. cadmium 32%, tin 68% 177(178) T, P Eutectic alloy lead 32%, tin 68% 177 T, P bismuth 12.8%, lead 49%, tin 38.2% 172 T, P potassium 80%, thallium 20% 165 T Chem. act bismuth 13.3%, lead 46%, tin 40.1% 165 T, P ∑? bismuth 10.5%, lead 42%, tin 47.5% 160 T, P bismuth 13.7%, lead 44.8%, tin 41.5% 160 T, P Eutectic alloy bismuth 16%, lead 36%, tin 48% 155 T, P bismuth 18.1%, lead 36.2%, tin 45.7% 151 T, P bismuth 25%, lead 50%, tin 25% 149 T, P bismuth 62.5%, cadmium 37.5% 149 T, P bismuth 19%, lead 38%, tin 43% 148 T, P bismuth 50%, lead 50% 145 T, P lead 32%, tin 50%, cadmium 18% 145 T, P bismuth 60%, cadmium 40% 144 T, P Eutectic alloy lead 42%, tin 37% 143 T, P ∑? cadmium 18.2%, lead 30.6%, tin 51.2% 142 8.8 T, P
How to determine a sample by density?
A method for determining the proportion of silver in an alloy was invented by Archimedes. The meaning of this non-destructive testing method is based on weighing the metal in water. In order to determine density, you need to know two quantities - volume and mass.
The weight of the product is determined by weighing on a scale. But how to determine the volume? To do this, we measure the volume of water displaced by the product. Divide mass by volume and get density. Using the table, we determine which metal sample the product belongs to.
Using the same method, you can distinguish other metals from each other. For example, the latest technology in the field of costume jewelry is the production of stainless steel products. To the untrained eye, it can be difficult to determine what kind of white metal a particular piece of jewelry is made of, but this can easily be determined by its density. Steel is much lighter than silver for the same volumes.
Melting alloys
When melted, it easily reacts with oxygen, so it is melted in a covered graphite crucible. The crucible is preheated to remove dirt and moisture. If a ceramic crucible is used, then coal is added to it during melting. Phosphorus or phosphorous copper may be added to prevent oxidation of the alloy.
If a graphite crucible is used, this is not necessary, since oxidation is minor and subsequent cleaning will remove it. The appearance of the surface of the silver when cast can provide vital information - at the right temperature it has a pinkish tint, the surface is clean and shiny. After melting, the top layer, which contains copper oxides, is removed. It is important not to overheat the alloy as this may affect its stability and uniformity.