The influence of carbon on the properties of steels
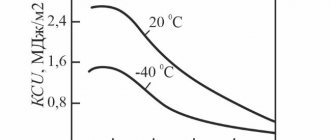
Carbon is the main strengthening element in all steels except austenitic stainless steels and some other high-alloy steels. The strengthening effect of carbon consists of solid solution strengthening and strengthening due to dispersed precipitation of carbides. With increasing carbon content in steel, its strength increases, but ductility and weldability decreases.
Carbon has a moderate tendency to macrosegregate during crystallization. Macrosegregation of carbon is usually more significant than that of all other alloying elements. Carbon has a strong tendency to segregate at defects in steels such as grain boundaries and dislocations. Carbide-forming elements can react with carbon to form “alloyed” carbides.
Stainless steels: how composition affects properties
Alloy steels occupy a significant share of the metallurgical products market.
These include the so-called “stainless steels” - a group of alloys characterized by increased resistance to corrosion. Since their appearance, the range of such steels has expanded to several hundred items. Therefore, a system for their classification and labeling was developed. It is worth noting that the name “stainless steel” does not entirely correctly reflect its properties. Any iron-carbon alloy is exposed to oxygen and aggressive substances, but for this to affect its performance properties, it takes different times. Therefore, stainless steels are more correctly called corrosion-resistant.
By composition
Chromium, nickel, vanadium, molybdenum, titanium and some others are used as alloying additives that increase the resistance of the iron-carbon alloy to rust formation.
Corrosion resistance is also increased by manganese and silicon introduced to deoxidize and neutralize sulfur. Based on the main alloying elements, stainless steels are classified as chromium, manganese, etc.
Some additives are used to impart special structural or technological properties to steels, for example, to crush carbides and increase impact toughness.
The basic alloying elements of stainless steel are chromium and nickel. They both enter into solid solution with iron and increase corrosion resistance.
When oxidized, they form a thin oxygen-impermeable film on the surface of a steel product, resistant to chemical, electrochemical and atmospheric influences. Nickel expands the austenite region in iron-carbon alloys.
Chromium narrows it, but is a carbide-forming element and binds carbon. The ratio of nickel and chromium has a decisive influence on impact strength, weldability and ability to withstand cold deformation.
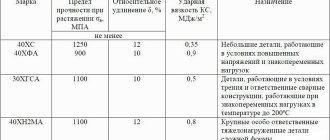
Carbon, as one of the essential components of steels, negatively affects corrosion resistance. However, the hardness and wear resistance of steel depends on its content. For example, 95Х18 has less pronounced corrosion-resistant properties compared to 40Х13, despite its higher chromium content.
By properties
A more clear idea of alloys is given by dividing them into groups according to properties:
- Corrosion resistant. Steels are highly resistant to atmospheric corrosion and can be used under normal conditions in a loaded state. Examples include stainless steel used for the manufacture of utensils and equipment for the food industry: 08Х18Н10, 20Х13, 30Х13.
- Heat resistant. A distinctive feature of such alloys is their high resistance to scale formation at high temperatures. Heat-resistant stainless steels are used for the manufacture of heat exchangers for boiler and pyrolysis plants (15Х28), valves for automobile and aircraft engines (40Х10С2М), parts for heating metallurgical furnaces (10Х23Н18).
- Heat resistant . A number of alloys have been developed that can operate under load at high temperatures without significant deformation and destruction. They use complex alloying systems (05Х27У5, 15Х12ВН14Ф, 37Х12Н8Г8МФБ). Steel type 20Х13 also has moderate heat resistance.
By structure
Based on their microstructure, stainless steels are divided into the following classes:
- austenitic;
- ferritic;
- martensitic;
In addition to them, there are intermediate groups:
- austenitic-ferritic;
- martensitic-ferritic;
- martensitic-carbide.
Heat treatment has a great influence on corrosion resistance, since it affects the phase composition of most stainless steels. Stability decreases when carbide heterogeneity occurs. This phenomenon is caused by the so-called intercrystalline corrosion.
When steels are heated to temperatures in the range of 500 – 800 °C, chains of carbides and areas with a reduced chromium content are formed at the grain boundaries. In the body of the grain, the content of alloying elements remains high. This type of corrosion is often observed in weld areas.
To combat this phenomenon, the steel composition is stabilized by introducing a small amount of titanium.
Austenitic steels
During crystallization, austenitic steels form a single-phase system with a face-centered crystal lattice. One of the most prominent representatives of the class is alloy 08Х18Н10.
Due to the high nickel content in stainless steels of this class (up to 30%), the austenite phase remains stable down to – 200 °C, and the carbon content does not exceed 0.12%. Steels with this structure are characterized by the absence of magnetic properties.
Most of them have good machinability.
Austenitic steels are necessarily subjected to heat treatment - hardening, tempering or annealing. The cooling rate practically does not change the hardness, but it affects the resistance to liquid and gaseous aggressive media, stabilizes the grain size and resistance to deformation.
Additional elements are introduced into the alloying systems of austenitic chromium-nickel steels:
- molybdenum – to prevent pitting and operation in reducing atmospheres
- titanium and niobium – for protection against intercrystalline corrosion.
- silicon – to increase acid resistance;
- manganese - to improve casting qualities.
Ferritic steels
This class includes chromium steels with low carbon content. They have a body-centered cubic lattice, which determines their magnetic properties.
Ferritic steels have lower corrosion resistance compared to austenitic steels and cannot be strengthened by heat treatment, but have higher technological properties. They are easier to machine and weld better, and their cost is significantly lower.
At temperatures of 300 – 400 °C, steels acquire high ductility, and they can be used to produce volumetric stamped parts of complex shapes.
chromium in such steels reaches 27%. Molybdenum, titanium and aluminum are used as stabilizing additives.
Martensitic steels
Alloys of this class contain at least 0.15% carbon and 11% chromium. Martensite has a microscopic needle-like structure and, when magnified, looks the same as carbon steel after hardening.
The crystal lattice has a tetragonal shape and is characterized by high internal stresses. This determines high strength properties and hardness. For example, for 40X13 it is up to 52 - 55 HRC.
Molybdenum, niobium, vanadium and tungsten are introduced as additional alloying elements. Martensitic steels, due to their high hardness, are difficult to cut and have low ductility.
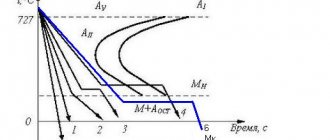
One of the main technological properties of corrosion-resistant steels with this structure is the ability to self-harden. Martensitic transformation occurs upon cooling in air. To increase heat resistance, steel after hardening is tempered with sorbitol or troostite.
The influence of manganese on the properties of steels
Manganese is present in almost all steels in amounts of 0.30% or more. Manganese is used to remove oxygen and sulfur from steel. It has less tendency to segregate than any other alloying element. Manganese has a beneficial effect on surface quality over the entire carbon content range, with the exception of very low carbon steels, and also reduces the risk of red brittleness. Manganese has a beneficial effect on the ductility and weldability of steels.
Manganese does not form its own carbide, but only dissolves in cementite and forms alloyed cementite in steels. Manganese promotes the formation of austenite and therefore expands the austenite region of the phase diagram. High manganese content (more than 2%) leads to an increased tendency to cracking and warping during hardening. The presence of manganese in steels encourages impurities such as phosphorus, tin, antimony and arsenic to segregate to the grain boundaries, causing temper brittleness.
Manganese in steel and alloys
Today, such an element as manganese is used very widely in steel and alloys ; one might even say that it is a metal that is constantly used in the production of steel. And although the ore, which was called manganese, has been used by man since the 13th century, this metal was discovered in it only in 1774. This discovery was made by the Swedish scientist K.-W. Scheele. But before the industrial use of manganese it was necessary to wait almost another hundred years.
In 1856, the English metallurgist R. Mushet received a patent for the following idea - to use manganese as a deoxidizer when melting steel. Indeed, by adding manganese to molten steel, ferum oxides are reduced, oxygen is removed, and the formation of ferum sulfides is prevented. The resulting manganese oxides and sulfides are easily separated from the metal and form slag. Moreover, for deoxidation it is enough to introduce about 6 kg of manganese per 1 ton of steel. The idea was so successful that it is still used in metallurgy to this day.
But it turned out that manganese is also good as an alloying additive to steel. In 1882, another English metallurgist R.-A. Hadfield proposed using steel containing 12-14% manganese for the manufacture of railway rails. Steel with this additive was characterized by high impact strength and wear resistance. This steel is believed to be the first alloy steel. Other positive effects discovered later when introducing manganese into steel include improved weldability, hardenability, cutting and pressure processing.
"Hadfield steel") deserves special attention
, containing 1.2% carbon and 13% manganese). It is used for the manufacture of parts subject to heavy wear - tracks of tracked vehicles, excavator buckets, rail switches, ball mills, crushers, etc. The high wear resistance of such steel is explained by the fact that under the action of high impact loads, the austenite of the surface layer turns into martensite. Technologically, after hardening, for example, the working elements of an excavator bucket, G13 steel acquires an austenitic structure, the hardness and wear resistance of which are low. However, as soon as the bucket is put into operation and begins to work intensively, crashing into the ground, the austenite, having received an impact, turns into martensite in the surface layer. The result is a remarkable set of properties - high hardness and wear resistance of the surface and high impact strength of the core. They say that steel is riveted. It is interesting that when the surface wears, the transformation of austenite into martensite continues and moves deeper into the part all the time during operation. The disadvantage of G13 steel is that it is difficult to machine.
Another interesting alloy based on manganese is the “silent alloy”
. This is an alloy containing 70% manganese and 30% cuprum. It does not make a ringing sound when you hit it.
Manganese is also used as an alloying element in bronzes and brasses. As in steels, it increases strength and elasticity. For example, you need a spring through which current must flow. Pure cuprum is not suitable for this - its elastic properties are low. But if we add 5% manganese to cuprum, we get good conductive spring bronze.
Or this alloy - 86.8% silver, 8.8% manganese, 4.4% aluminum. It is used to make permanent magnets. How so? – you will be surprised. Indeed, all three elements have nothing to do with ferromagnets. However, as part of the alloy, the resulting crystal lattice has ferromagnetic properties.
Manganese compounds are widely used in various technologies - both as coloring agents and as an oxidizing agent in organic compounds, in the production of glass, and in the ceramic industry. And how many uses in everyday life does the ordinary “potassium permanganate” - potassium permanganate have!
After all of the above, it is not surprising that manganese is one of the most used metals, and its global production amounts to millions of tons.
The influence of silicon on the properties of steels
Silicon is one of the main deoxidizing agents used in steel smelting. Therefore, the silicon content determines the type of steel produced. Mild carbon steels can contain silicon up to a maximum of 0.60%. Semi-quiet steels may contain moderate amounts of silicon, for example 0.10%.
Silicon is completely dissolved in ferrite at a silicon content of up to 0.30%. It increases the strength of ferrite without almost reducing its ductility. When the silicon content is above 0.40% in general purpose carbon steel, a significant decrease in ductility occurs.
In combination with manganese or molybdenum, silicon provides higher hardenability of steel. The addition of silicon to chromium-nickel austenitic steels increases their resistance to stress corrosion. In thermally hardenable steels, silicon is an important alloying element; it increases the ability of steels to be thermally hardened and their wear resistance, increases the elastic limit and yield strength. Silicon does not form carbides and does not contain cementite or other carbides. It dissolves in martensite and slows down the decomposition of alloyed martensite up to 300 °C.
Influence of alloying elements and impurities on weldability
Influence of alloying elements and impurities on weldability
Alloying elements include: chromium, nickel, molybdenum, vanadium, tungsten, titanium, as well as manganese and silicon at a certain content.
Chromium in low-carbon steels is contained in the range up to 0.300, in structural steels 0.7 - 3.50, in chromium steels l2 - 180~0, in chromium-nickel steels 9 - 3500. When welding, chromium forms chromium carbides, which worsen the corrosion resistance of steel and sharply increase hardness - in heat-affected zones; promotes the formation of refractory oxides, which complicate the welding process.
Nickel in low-carbon steels ranges from 0.2-0.3%, in structural steels 1 - 5%, in alloyed steels 8 - 35%. In some alloys the nickel content reaches 85%. Nickel increases the plastic and strength properties of steel, refines the grains without impairing weldability.
Molybdenum in steel is limited to 0.15 - 0.8%. It increases the load-bearing capacity of steel under shock loads and high temperatures, and refines the grain. It promotes the formation of cracks in the deposited metal and heat-affected zones; When welding, it actively oxidizes and burns out.
Vanadium in special steels is contained in the range of 0.2 - 0.8%, in die steels 1 - 1.5%. It promotes the hardening of steel, which makes welding difficult. During the welding process, it actively oxidizes and burns out.
Tungsten in tool and die steels ranges from 0.8 to 18%. Tungsten sharply increases the hardness of steel and its performance at high temperatures (red resistance), but complicates the welding process, as it is highly oxidized.
Titanium and niobium are introduced into stainless and heat-resistant steels to increase corrosion properties (0.5 - 1.0%). When welding stainless steels of the X18N9 type, niobium promotes the formation of hot cracks.
Carbon is one of the most important impurities, determining the strength, toughness, hardenability and especially weldability of steel. Carbon content in conventional structural steels up to 0.25% does not impair weldability. At higher contents, the weldability of steel sharply deteriorates, since hardening structures are formed in heat-affected zones, leading to cracks. An increased carbon content in the filler material causes porosity of the weld metal during welding.
Manganese is of 0.3 - 0.8%. Manganese does not complicate the welding process. When welding medium-manganese steels (1.8 - 2.5% Mn), there is a danger of cracks due to the fact that manganese increases the hardenability of steel. In steels of the G13L type with a manganese content in the range of 11 - 16%, intense burnout of manganese occurs during welding, which requires special measures to prevent.
Silicon is found in steel in the range of 0.02 - 0.3%. It does not cause any difficulties during welding. In special steels with a silicon content of 0.8 - 1.5%, welding conditions deteriorate due to the high fluidity of silicon steel and the formation of refractory silicon oxides.
Welding carbon steels
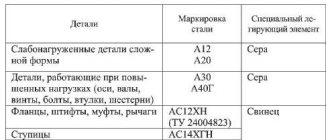
Carbon steel is an alloy of iron and carbon with minor amounts of silicon, manganese, phosphorus and sulfur. In carbon steel, unlike stainless steel, there are no alloying elements (molybdenum, chromium, manganese, nickel, tungsten). The properties of carbon steel vary greatly depending on a slight change in carbon content. As the carbon content increases, the hardness and strength of the steel increase, while the toughness and ductility decrease. With a carbon content of more than 2.14%, the alloy is called cast iron. Carbon increases the ability of steel to be hardened. Steel with a carbon content (0.25–0.55%) is subject to quenching and tempering, which significantly increases its hardness and wear resistance. These qualities of steel are used in the production of mechanism parts, axle shafts, gears, housings, sprockets and other parts that require increased wear resistance. Often, welding becomes the only technology for the manufacture and repair of machine parts, frames of production equipment, etc.