General information about steel hardening technology
The main goals achieved by the hardening + tempering complex:
- increased hardness;
- increasing strength characteristics;
- reduction of ductility to an acceptable value;
- the possibility of using hollow products instead of solid ones, which allows reducing the weight of the metal product and the metal intensity of the production process.
Main stages of hardening:
- heating to temperatures at which the structural state of the metal changes;
- shutter speed set in the technological map;
- cooling at a rate that ensures the formation of a given crystal structure.
After hardening, tempering is carried out, which consists of heating the metal to temperatures below the line of phase transformations, with a further slow decrease in temperature. The result of heat treatment is influenced by:
- heating temperature;
- rate of temperature rise;
- holding period at quenching temperatures;
- cooling medium and rate of temperature decrease.
The key parameter is the heating temperature, on which the restructuring and formation of a new structural lattice depends. Based on the depth of action, hardening is divided into volumetric and surface. In mechanical engineering, volumetric hardening is usually used, after which the hardness of the surface and core differs slightly. Surface heat treatment is in demand for parts for which high surface hardness and a viscous core are important.
What steels are hardened?
Not all steel grades can be hardened. Grades with a carbon content below 0.4% practically do not change hardness at quenching temperatures, so this method is not used for them. Hardening technology is most often used for tool steels.
Table of correct quenching and tempering conditions for certain types of tool steels
| steel grade | Steel hardening temperature | Cooling medium after quenching heating | Holiday temperature | Cooling environment after tempering |
| U7 | 800°C | water | 170°C | water, oil |
| U7A | 800°C | water | 170°C | water, oil |
| U8, U8A | 800°C | water | 170°C | water, oil |
| U10, U10A | 790°C | water | 180°C | water, oil |
| U11, U12 | 780°C | water | 180°C | water, oil |
| P9 | 1250°C | oil | 580°C | air in the oven |
| P18 | 1250°C | oil | 580°C | air in the oven |
| ШХ6 | 810°C | oil | 200°C | air |
| ШХ15 | 845°C | oil | 400°C | air |
| 9ХС | 860°C | oil | 170°C | air |
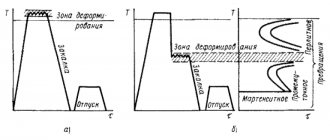
Types of hardening - with and without polymorphic transformation
Hardening of steels proceeds with a polymorphic transformation, non-ferrous metals and alloys - without them.
Hardening of steels with polymorphic transformation
In carbon steels, when temperatures rise above a certain level, a series of phase transformations occur, causing changes in the crystal lattice. At critical temperatures, the value of which depends on the percentage of carbon, the decomposition of iron carbide occurs and the formation of a solution of carbon in iron, called austenite. With slow cooling, austenite gradually disintegrates, and the crystal lattice acquires its original state. If carbon steels are cooled at a high speed, then, depending on the quenching mode, various phase states are formed in them, the strongest of which is martensite.
To obtain a martensitic structure, hypoeutectoid steels (up to 0.8% C) are heated to temperatures above the Ac3 point by 30-50°C, for hypereutectoid steels - 30-50° above Ac1. Using this technology, metal-cutting tools are hardened and products are strengthened, which are subject to friction during operation: gears, shafts, races, bushings. When heated to lower temperatures, the structure of hypoeutectoid steels, along with martensite, retains softer ferrite, which reduces the hardness of the metal and worsens its mechanical characteristics after tempering. Such hardening of steel is called incomplete and in most cases is a defect. But it can be used in some cases to avoid cracks.
Hardening without polymorphic transformation
Hardening without polymorphic transformation occurs in non-ferrous metals and alloys that have limited solubility of secondary phases at ordinary temperatures, in which polymorphic transformations do not occur at high temperatures. When temperatures increase above the solidus line (this is the line below which only the solid phase is located), the secondary phases completely dissolve. During rapid cooling, secondary phases do not separate out, since this requires a certain time. After such heat treatment, the non-ferrous alloy is thermodynamically unstable, so over time it begins to decompose with the gradual release of a secondary phase. This decomposition process, which occurs under natural conditions, is called natural aging, and when heated, it is called artificial aging. As a result of aging, an equilibrium structure is obtained. The characteristics of the material depend on the selected process mode.
Hardening of non-ferrous metals and alloys, unlike carbon steels, often does not lead to an increase in strength. Copper-based alloys, for example, often become more ductile after such maintenance. For such materials, tempering is usually used, which relieves stress after casting, rolling, stamping, forging or pressing.
Purpose of heat treatment
Heat treatment of steel is carried out at temperatures close to critical points. Here's what happens:
- secondary crystallization of the alloy;
- transition of gamma iron to the alpha iron state;
- transition of large particles into plates.
The internal structure of a two-phase mixture directly affects performance and ease of processing.
Formation of structures depending on cooling intensity
The main purpose of heat treatment is to give steels:
- In finished products:
- strength;
- wear resistance;
- corrosion resistance;
- heat resistance.
- In blanks:
- relief of internal stresses after casting;
- stamping (hot, cold);
- deep drawing;
Heat treatment is applied to the following types of steels:
- Carbon and alloyed.
- With varying carbon contents, from low carbon 0.25% to high carbon 0.7%.
- Structural, special, instrumental.
- Any quality.
Steel hardening methods
The hardening method is chosen depending on the chemical composition of the steel and the planned properties.
Hardening with cooling in one environment
The rate of cooling of steel after hardening depends on the environment in which it is carried out. The highest speed is provided by cooling in water. This method is used for medium-carbon low-alloy steels and some grades of corrosion-resistant steels. When the carbon content is more than 0.5% C and high alloying, water is not used as a cooling medium, since such alloys become cracked or completely destroyed.
Intermittent quenching in two cooling media
Step hardening is used for parts made of complex alloy steels. Large parts, after heating, are dipped in water for several minutes, and then cooled in oil to +320...300°C, after which they are left in the air. When cooled in oil to room temperature, the hardness of the product decreases significantly.
Isothermal maintenance
Hardening of high-carbon grades is a complex process consisting of normalization followed by heating to the hardening temperature. The heated parts are lowered into a bath of saltpeter, heated to temperatures of +320...+350°C, and kept.
Svetlaya TO
This heat treatment is used for high-alloy steels and consists of heating them in an environment of inert gases or in a vacuum, which ensures a light-colored metal surface. Light hardening is used in mass production of standard products.
Heat treatment with self-tempering
At a high cooling rate, heat remains inside the part, which, when gradually released, relieves stress on the internal structure. This process can only be entrusted to specialists who can accurately calculate the time the product is in the cooling environment.
Jet
Cooling is carried out with an intense stream of water. This process is used when it is necessary to harden individual parts of products.
Lecture No. 13. Heating of metal. Metal heating calculations.
However, complete uniform heating of the metal before pressure treatment is not required, since during its transportation from the furnace to the mill or press and rolling (forging), temperature equalization inevitably occurs across the cross-section of the ingots and billets due to the release of heat to the environment from their surface and thermal conductivity into the metal. Based on this, the permissible temperature difference across the cross section is usually taken according to practical data during heating before pressure treatment within the following limits: for high-alloy steels ∆ Tcon
= 100δ;
for all other steel grades ∆ Tkon
= 200δ for δ <0.1 m and ∆
Tkon
= 300δ for δ > 0.2 m. Here δ is the heated thickness of the metal.
In all cases, the temperature difference across the thickness of the workpiece at the end of its heating before rolling or forging should not exceed 50 °C, and when heating for heat treatment, 20 °C, regardless of the thickness of the product. When heating large ingots, it is allowed to release them from the furnace at ∆ Tkon
<100 °C.
Another important task of metal heating technology is to ensure uniform temperature distribution over the entire surface of workpieces or products by the time they are unloaded from the furnace. The practical necessity of this requirement is obvious, since with significant uneven heating over the surface of the metal (even when the required temperature difference across the thickness is achieved), defects such as uneven profile of the finished rolled product or different mechanical properties of the product subjected to heat treatment are inevitable.
Ensuring temperature uniformity over the surface of the heated metal is achieved through the correct selection of a furnace for heating a certain type of workpiece or product and the appropriate placement of heat-generating devices in it, creating the necessary temperature field in the working space of the furnace, the relative position of the workpieces, etc.
Heating duration
to the final temperature is also the most important indicator, since the productivity of the furnace and its dimensions depend on it. At the same time, the duration of heating to a given temperature determines the heating rate, i.e., the change in temperature at some point of the heated body per unit time. Typically, the heating rate changes as the process progresses, and therefore a distinction is made between the heating rate at a certain point in time and the average heating rate over the time interval under consideration.
The faster the heating is carried out (i.e., the higher the heating rate), the obviously higher the productivity of the furnace, all other things being equal. However, in a number of cases, the heating rate cannot be chosen as high as desired, even if the conditions of external heat transfer allow it to occur. This is due to certain restrictions imposed by the conditions of the processes that accompany the heating of metal in furnaces and are discussed below.
Processes that occur when metal is heated. When a metal is heated, its enthalpy changes, and since in most cases heat is supplied to the surface of ingots and workpieces, their external temperature is higher than the temperature of the internal layers. As a result of thermal expansion of different parts of a solid by different amounts, stresses arise, called thermal.
Another group of phenomena is associated with chemical processes on the surface of the metal when heated. The metal surface, which is at a high temperature, interacts with the environment (i.e., combustion products or air), as a result of which a layer of oxides is formed on it. If any elements of the alloy interact with the environment surrounding the metal to form a gas phase, then the surface is depleted of these elements. For example, the oxidation of carbon in steel when it is heated in furnaces causes surface decarbonization.
Thermal stress
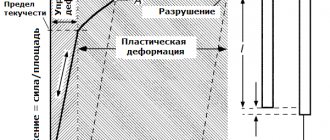
As noted above, in the cross section of ingots and billets, when they are heated, an uneven temperature distribution occurs and, therefore, different parts of the body tend to change their size to different degrees. Since in a solid body there are connections between all its individual parts, they cannot be independently deformed in accordance with the temperatures to which they are heated. As a result, thermal stresses arise due to temperature differences. The outer, more heated layers tend to expand and are therefore in a compressed state. The inner, cooler layers are subject to tensile forces. If these stresses do not exceed the elastic limit of the heated metal, then as the temperature equalizes over the cross section, the thermal stresses disappear.
All metals and alloys have elastic properties up to a certain temperature (for example, most grades of steel up to 450-500 ° C). Above this certain temperature, metals pass into a plastic state and the thermal stresses that arise in them cause plastic deformation and disappear. Consequently, temperature stresses should be taken into account when heating and cooling steel only in the temperature range from room temperature to the point of transition of a given metal or alloy from an elastic to a plastic state. Such stresses are called vanishing or temporary.
In addition to temporary ones, there are residual temperature stresses that increase the risk of destruction when heated. These stresses occur if the ingot or workpiece has previously been subjected to heating and cooling. When cooling, the outer layers of the metal (colder) reach the temperature of transition from the plastic to elastic state earlier. As further cooling occurs, the inner layers are exposed to tensile forces, which do not disappear due to the low ductility of the cold metal. If this ingot or billet is heated again, then the temporary stresses arising in them will be superimposed with the same sign on the residual ones, which will aggravate the danger of cracks and ruptures.
In addition to temporary and residual temperature stresses, stresses caused by structural changes in volume also arise during heating and cooling of alloys. But since these phenomena usually take place at temperatures exceeding the boundary of transition from an elastic to a plastic state, the structural stresses are dissipated due to the plastic state of the metal.
The relationship between strains and stresses is established by Hooke's law
σ= ( Тср-Т
)
where β is the linear expansion coefficient; Tsr
- average body temperature;
T
is the temperature in a given section of the body;
E
- modulus of elasticity (for many grades of steel, the value of
E
decreases from (18÷22).104 MPa to (14÷17).104 MPa with increasing temperature from room temperature to 500 °C; σ - stress; v - Poisson's ratio ( for steel v ≈ 0.3).
Of great practical interest is finding the maximum permissible temperature difference across the cross section of the body ∆Tdop = Tpov - Tcen. The most dangerous in this case are tensile stresses, so they should be taken into account when calculating the permissible temperature difference. As a strength characteristic, the value of the alloy's tensile strength σv should be taken.
Then, using solutions to heat conduction problems (see Chapter 16) and imposing expression (21-1) on them, for the case of a regular mode of the second kind, one can, in particular, obtain:
for uniformly and symmetrically heated endless plate
∆T
additional = 1.5 (1 - v) σ in /( );
for a uniformly and symmetrically heated endless cylinder
∆T
additional = 2 (1 - v) σ in /( ).
The permissible temperature difference, found using formulas (21-2) and (21-3), does not depend on the size of the body and its thermophysical characteristics. Body dimensions have an indirect effect on the value of ∆ T
additional, since the residual stresses in larger bodies are greater.
Oxidation and decarbonization of the surface when heated. Oxidation of ingots and billets when heated in furnaces is an extremely undesirable phenomenon, since it results in irreversible loss of metal. This leads to very large economic damage, which becomes especially obvious if we compare the cost of metal losses during oxidation with other processing costs. So, for example, when heating steel ingots in heating wells, the cost of the metal lost with scale is usually higher than the cost of the fuel spent heating this metal and the cost of electricity spent on rolling it. When heating billets in the furnaces of long-rolling shops, losses from scale are somewhat lower, but they are still quite large and are comparable in cost to fuel costs. Since on the way from the ingot to the finished product the metal is usually heated several times in different furnaces, losses due to oxidation are quite significant. In addition, the higher hardness of oxides compared to metal leads to increased tool wear and increases the percentage of defects during forging and rolling.
The lower thermal conductivity of the oxide layer formed on the surface of the metal increases the heating time in furnaces, which entails a decrease in their productivity, all other things being equal, and crumbling oxides form slag build-ups on the furnace floor, making operation difficult and causing increased consumption of refractory materials.
The appearance of scale also makes it impossible to accurately measure the temperature of the metal surface, set by technologists, which complicates the control of the thermal regime of the furnace.
The above-mentioned interaction with the gas environment in the furnace of any alloy element is of practical importance for steel. A decrease in carbon content in it causes a decrease in hardness and tensile strength. To obtain the specified mechanical properties of the product, it is necessary to remove the decarbonized layer (reaching 2 mm), which increases the complexity of processing as a whole. Decarbonization of those products that are subsequently subject to surface heat treatment is especially unacceptable.
The processes of oxidation of the alloy as a whole and its individual impurities when heated in furnaces should be considered together, since they are closely related to each other. For example, according to experimental data, when steel is heated to a temperature of 1100°C and higher in a conventional furnace atmosphere, oxidation occurs faster than surface decarburization, and the resulting scale plays the role of a protective layer that prevents decarburization. At lower temperatures, the oxidation of many steels (even in a strongly oxidizing environment) is slower than decarburization. Therefore, steel heated to a temperature of 700-1000 °C can have a decarbonized surface. This is especially dangerous since the temperature range of 700–1000 °C is typical for heat treatment.
Metal oxidation.
Oxidation of alloys is the process of interaction of oxidizing gases with their base and alloying elements. This process is determined not only by the rate of chemical reactions, but also by the patterns of formation of the oxide film, which, as it grows, isolates the metal surface from the effects of oxidizing gases. Therefore, the growth rate of the oxide layer depends not only on the course of the chemical process of steel oxidation, but also on the conditions of movement of metal ions (from the metal and internal layers of oxides to the external ones) and oxygen atoms (from the surface to the internal layers), i.e. on the conditions of flow physical process of two-way diffusion.
The diffusion mechanism of the formation of iron oxides, studied in detail by V.I. Arkharov, determines the three-layer structure of the scale layer formed when steel is heated in an oxidizing environment. The inner layer (adjacent to the metal) has the highest iron content and consists mainly of FeO (wustite): Fe B V202 C| FeCX The melting point of wustite is 1317 °C. The middle layer - magnetite Fe304, which has a melting point of 1565 ° C, is formed during the subsequent oxidation of wustite: 3FeO C 1/202 ift Fes04. This layer contains less iron and, compared to the inner layer, is enriched with oxygen, although not to the same extent as the most oxygen-rich hematite Fe208 (melting point 1538 ° C): 2Fe304 -f V202 - C 3Fe2Os. The composition of each layer is not constant over the cross section, but gradually changes due to impurities of more (closer to the surface) or less (closer to the metal) oxygen-rich oxides.
The oxidizing gas when heated in furnaces is not only free oxygen, but also bound oxygen, which is part of the products of complete combustion of fuel: CO2 H20 and S02. These gases, like O2, are called oxidizing gases in contrast to reducing gases: CO, H2 and CH4, which are formed as a result of incomplete combustion of fuel. The atmosphere in most fuel furnaces is a mixture of N2, CO2, H20 and S02 with a small amount of free oxygen. The presence of a large amount of reducing gases in the furnace indicates incomplete combustion and unacceptable fuel use. Therefore, the atmosphere of conventional fuel furnaces always has an oxidizing character.
The oxidizing and reducing ability of all of the listed gases in relation to the metal depends on their concentration in the furnace atmosphere and on the temperature of the metal surface. The strongest oxidizing agent is O2, followed by H2O, and CO2 has the weakest oxidizing effect. Increasing the proportion of neutral gas in the furnace atmosphere reduces the oxidation rate, which largely depends on the content of H2O and SO2 in the furnace atmosphere. The presence of even very small amounts of SO2 in furnace gases sharply increases the rate of oxidation, since low-melting compounds of oxides and sulfides are formed on the surface of the alloy. As for H2S, this compound can be present in a reducing atmosphere and its effect on the metal (along with SO2) leads to an increase in the sulfur content in the surface layer. In this case, the quality of the metal is greatly deteriorated, and sulfur has a particularly harmful effect on alloyed steels, since they absorb it to a greater extent than simple carbon steels, and nickel forms a low-melting eutectic with sulfur.
The thickness of the resulting layer of oxides on the surface of the metal depends not only on the atmosphere in which the metal is heated, but on a number of other factors, which primarily include the temperature and duration of heating. The higher the temperature of the metal surface, the higher the rate of its oxidation. However, it has been found that the growth rate of the oxide layer increases faster after reaching a certain temperature. Thus, the oxidation of steel at temperatures up to 600°C occurs at a relatively low rate, and at temperatures above 800-900°C the growth rate of the oxide layer increases sharply. If we take the oxidation rate at 900 °C as one, then at 950 °C it will be 1.25, at 1000 °C - 2, and at 1300 - 7.
The length of time the metal remains in the furnace has a very strong influence on the amount of oxides formed. Increasing the duration of heating to a given temperature leads to growth of the oxide layer, although the oxidation rate decreases with time due to the thickening of the resulting film and, consequently, a decrease in the density of the diffusion flux of iron ions and oxygen atoms through it. It has been established that if the thickness of the oxidized layer is δ1 at heating time t1
then at heating time
t2
to the same temperature, the thickness of the oxidized layer will be equal to:
δ2= δ1/( t1
/
t2
)1/2.
The duration of heating the metal to a given temperature can be reduced, in particular, as a result of increasing the temperature in the working chamber of the furnace, which leads to more intense external heat transfer and, thus, helps to reduce the thickness of the oxidized layer.
It has been established that factors influencing the intensity of oxygen diffusion to the surface of the heated metal from the furnace atmosphere do not have a significant effect on the growth of the oxide layer. This is due to the fact that diffusion processes in the hard surface itself proceed slowly and they are decisive. Therefore, the speed of gas movement has virtually no effect on surface oxidation. However, the pattern of movement of combustion products as a whole can have a noticeable effect, since local overheating of the metal, caused by an uneven temperature field of the gases in the furnace (which can be caused by an excessively large angle of inclination of the burners, their incorrect placement along the height and length of the furnace, etc.) , inevitably lead to local intense oxidation of the metal.
The conditions for moving heated workpieces inside the furnaces and the composition of the heated alloy also have a noticeable effect on the rate of its oxidation. Thus, when moving metal in a furnace, mechanical peeling and separation of the resulting oxide layer can occur, which contributes to faster subsequent oxidation of unprotected areas.
The presence of certain alloying elements in the alloy (for example, for steel Cr, Ni, Al, Si, etc.) can ensure the formation of a thin and dense, well-adhering film of oxides, which reliably prevents subsequent oxidation. Such steels are called heat-resistant and have good resistance to oxidation when heated. In addition, steel with a higher carbon content is less susceptible to oxidation than low-carbon steel. This is explained by the fact that in steel some of the iron is in a state bound to carbon, in the form of iron carbide Fe3C. The carbon contained in steel, oxidizing, turns into carbon oxide, diffusing to the surface and preventing the oxidation of iron.
Decarburization of the surface layer of steel
. Decarburization of steel during heating occurs as a result of the interaction of gases with carbon, which is either in the form of a solid solution or in the form of iron carbide Fe8C. Decarburization reactions resulting from the interaction of various gases with iron carbide can proceed as follows:
Fe3C + H2O = 3Fe + CO +
H2; 2Fe3C + O2 = 6Fe + 2CO;
Fe3C + CO2 = 3Fe + 2CO; Fe3C + 2H2 = 3Fe + CH4.
Similar reactions occur when these gases interact with carbon in solid solution.
The rate of decarbonization is determined mainly by the process of two-way diffusion, which occurs under the influence of the difference in concentrations of both media. On the one hand, decarburizing gases diffuse to the surface layer of steel, and on the other, the resulting gaseous products move in the opposite direction. In addition, carbon from the inner layers of the metal moves to the surface decarbonized layer. Both the rate constants of chemical reactions and the diffusion coefficients increase with increasing temperature. Therefore, the depth of the decarbonized layer increases with increasing heating temperature. And since the density of the diffusion flux is proportional to the difference in the concentrations of the diffusing components, the depth of the decarbonized layer is greater in the case of heating high-carbon steel than in the case of heating low-carbon steel. Alloying elements contained in steel also play a role in the decarburization process. Thus, chromium and manganese reduce the carbon diffusion coefficient, while cobalt, aluminum and tungsten increase it, respectively preventing or promoting the decarbonization of steel. Silicon, nickel and vanadium do not have a significant effect on decarburization.
Gases that are part of the furnace atmosphere and cause decarbonization include H20, CO2, O2 and H2. H20 has the strongest decarbonizing effect on steel, and H2 has the weakest. At the same time, the decarbonization ability of CO2 increases with increasing temperature, and the decarbonization ability of dry H2 decreases. Hydrogen in the presence of water vapor has a very strong decarburizing effect on the surface layer of steel.
Protection of steel from oxidation and decarburization.
The harmful effects of oxidation and decarbonization of metal during heating on its quality require the adoption of measures to prevent these phenomena. The most complete protection of the surface of ingots, billets and parts is achieved in furnaces, where exposure to oxidizing and decarbonizing gases is excluded. These furnaces include salt and metal baths, as well as furnaces where heating is carried out in a controlled atmosphere. In furnaces of this type, either the heated metal is isolated from gases, usually closed with a special sealed muffle, or the flame itself is placed inside the so-called radiant tubes, the heat from which is transferred to the heated metal without its contact with oxidizing and decarbonizing gases. The working space of such furnaces is filled with special atmospheres, the composition of which is selected depending on the heating technology and alloy grade. Protective atmospheres are prepared separately in special installations.
There is also a known method for creating a weakly oxidizing atmosphere directly in the working space of furnaces, without muffling metal or flame. This is achieved due to incomplete combustion of fuel (with an air consumption coefficient of 0.5–0.55). The composition of the combustion products includes CO and Na along with the products of complete combustion of CO2 and H2O. If the CO/CO2 and H2/H2O ratio is not less than 1.3, then heating of the metal in such an environment occurs almost without oxidation of its surface.
Reducing the oxidation of the metal surface when heating it in open-flame fuel furnaces (which make up the majority of the furnace fleet of metallurgical and machine-building plants) can also be achieved by reducing the duration of its stay at a high surface temperature. This is achieved by choosing the most rational heating mode for the metal in the furnace.
Calculations of metal heating in furnaces are performed to determine the temperature field of an ingot, billet or finished product, based on the conditions dictated by the technological purpose of heating. In this case, the restrictions imposed by the processes occurring during heating are taken into account, as well as the laws of the selected heating mode. The problem of determining the heating time to a given temperature is often considered, provided that the required uniformity is ensured by the end of its stay in the furnace (the latter in the case of massive bodies). In this case, changes in the temperature of the heating medium are usually set by the law, choosing a heating mode depending on the degree of thermal massiveness of the metal. To determine the degree of thermal massiveness and for the subsequent calculation of heating, the question of the heated thickness of the ingot or workpiece is very important.
⇐ Previous2Next ⇒
Recommended pages:
Equipment for hardening
The equipment is divided into two main groups - heating units and cooling baths. At modern enterprises, the following are used to obtain quenching temperatures:
- thermal muffle furnaces;
- induction heating equipment;
- installations for heating in melts;
- laser heating devices;
- gas plasma devices.
The first three types of installations are in demand for volumetric hardening, the last three - for the surface process.
Quenching equipment is steel containers, graphite crucibles, furnaces that contain molten metals or salts. Quenching baths for liquid media are equipped with heating and cooling systems. Their design may include special mixers for mixing liquid media and eliminating the steam jacket.
Hardening process technology
Heating and holding
The heating temperature of steel during hardening depends on its chemical composition. In general, there is a pattern - the lower the percentage of carbon, the higher the heating temperature should be. Lowering the heating temperature leads to the fact that the desired structure does not have time to form. Consequences of overheating:
- decarbonization;
- surface oxidation;
- increase in internal tension;
- change in structural components.
Products of complex shapes are preheated. To do this, they are dipped two or three times in salt baths for several minutes or kept for a short time in ovens heated to temperatures of +400...500°C. The holding period is determined by the dimensions of the product and their quantity in the oven. All parts of the product must be heated evenly.
Table of hardening temperatures for various steel grades
| Brand | Temperature, °C | Brand | Temperature, °C |
| 15G | 800 | 50G2 | 805 |
| 65G | 815 | 40ХГ | 870 |
| 15X, 20X | 800 | 3Х13 | 1050 |
| 30Х, 35Х | 850 | 35ХГС | 870 |
| 40Х, 45Х | 840 | 30ХГСА | 900 |
| 50X | 830 |
The heating temperature is measured using pyrometers - contact and non-contact, infrared devices.
Cooling
For cooling, water is used - pure or with salts dissolved in it, alkaline solutions. For alloy steels, blowing or cooling in mineral oils is used. In isothermal and stepwise processes, molten salts, alkalis and metals are used for cooling. Such environments can alternate with each other.
Vacation
Depending on the required temperature, tempering is carried out in oil, alkaline or saltpeter baths, furnaces with forced circulation of air flows, and hot sand.
Low tempering, carried out at +150...+200°C, serves to eliminate internal stresses, slightly increase ductility and toughness without significantly deteriorating hardness. Low tempering is in demand for measuring and metalworking tools, and other parts that must combine hardness and wear resistance.
For high-speed steels, tempering is carried out at temperatures of +550...580°C. This procedure is called secondary hardening because it leads to an additional increase in hardness.
Cooling methods
By cooling steel to different temperatures and at different speeds, it is possible to obtain different structures of its crystal lattice with elements of different sizes and shapes. The combination of these characteristics with the chemical composition determines its performance qualities such as hardness, fragility, viscosity, strength, elasticity, etc. Therefore, there are many cooling technologies and their varieties, among which the following technological groups can be distinguished:
- Cooling in one component. The product is immersed in liquid and remains in it until it cools completely.
- Intermittent hardening in two coolers. The product is first placed in a fast-cooling liquid, and after reaching a given temperature, it is transferred to a slow-cooling environment.
- Jet cooling. The heated part is intensively irrigated with a coolant flow (see photo below).
- Airflow The surface of the product is blown with a stream of air or inert gas.
In the practical application of hardening, all these types of cooling can have different variations or be combined with each other.
Cooling media
Water is usually used as a coolant when hardening carbon steels: both pure and in the form of aqueous solutions (salt and alkaline). Alloy steels require a lower cooling rate, so mineral oils and air are used for them. During stepwise and isothermal hardening, the cooling medium is molten salts, alkalis and metals. In some types of hardening, the cooling media alternate to obtain the required steel structure.
| № | Structure | Cooling medium | Hardness (HBW) |
| 1 | Martensite | Cold water | 500÷750 |
| 2 | Troostitis | Oil | 350÷500 |
| 3 | Sorbitol | Air | 250÷350 |
| 4 | Perlite | As the oven cools down | 150÷250 |
The influence of cooling speed on the final result
When hardening steel, cooling must occur at a rate that prevents the decomposition of austenite into ferrite and iron carbide, which begins to occur at temperatures below 650 °C. Further reduction in temperature should be carried out more slowly, since such a speed ensures a decrease in the internal stresses of the steel. Rapid and complete cooling in cold water produces martensite, which has maximum hardness but is quite brittle. With a rapid decrease in temperature by 200÷300 °C, the decomposition of austenite stops, and further slower cooling forms phase states in the steel with lower hardness, but with increased strength and wear resistance. The cooling rate is controlled by the type of quenching medium used and its temperature (see table below).
| № | Cooling medium | Cooling rate (deg/sec) |
| 1 | Air | 5 |
| 2 | Mineral oil | 150 |
| 3 | Water at room t° | 700 |
| 4 | Water at 80 °C | 1400 |
| 5 | 10% sodium chloride solution | 2100 |
| 6 | 10% sodium hydroxide solution | 1600 |
Possible defects after hardening
Heating, holding, cooling and tempering of steel are carried out in accordance with technological maps developed by specialists. Violation of the developed and approved technical process and/or heterogeneity of the workpiece structure can cause various defects. Among them:
- Uneven heating and/or cooling. They lead to deformations and the formation of cracks, non-uniform composition and non-uniform mechanical characteristics.
- Burnout. Occurs due to the penetration of oxygen molecules into the metal surface. As a result, oxides are formed that change the performance characteristics of the surface layer. This defect occurs due to the burning of carbon from the steel caused by excess oxygen in the furnace.
- Water entering the oil cooling bath. This violation of the technical process leads to the appearance of cracks in the product.
All of the above defects are irreparable.
Defects during hardening of steel
The cause of defects during steel hardening is a number of physical and chemical factors that arise when there is a deviation from the specified parameters of the thermal process or due to the heterogeneity of the workpiece being hardened. Uneven heating or cooling of the product can lead to its deformation and internal cracks. The same reason can cause uneven phase transformations in different parts of the product, as a result of which the metal will have a structure that is heterogeneous in composition and hardness. Burnout of steel occurs due to the penetration of oxygen into the surface layer of the metal, which leads to the formation of oxides that separate its structural elements and change the physical properties of the surface layer. The reason for decarburization during steel hardening is the burnout of carbon when excess oxygen enters the furnace. These types of defects are irreparable, and the only way to deal with them is to check the tightness of the furnace or hardening in a vacuum and inert gases.
Scales and a critical decrease in carbon concentration during heating
Even a small concentration of oxygen in a hardening furnace leads to the appearance of surface scale, which is a consequence of the oxidation of the metal during its heat treatment. The same reason can cause a decrease in the amount of carbon in the surface layer of the workpiece. It is possible to completely get rid of such phenomena only by using vacuum furnaces, which provide so-called light hardening, as well as by heating the product in a nitrogen or argon environment. To minimize oxidation and decarburization, the hardening furnace must be as sealed as possible, which to some extent limits the flow of oxygen into its working space.
For hardening metals, it is recommended to use transformer or industrial oil I-20. It is not easy for a private owner to get it, so I would like to hear in the comments to this article your opinion on the possibility of using used car oil or other automobile oil .