Steel is hardened to increase its hardness, strength and wear resistance. This is one of the types of heat treatment in which the metal is first heated to temperatures that change its structural state, and then cooled so that it acquires the required physical and chemical composition and the necessary crystal structure. There are many ways to harden steel, leading to different results, but they all consist of two main cycles: heating to a critical point and cooling at a certain rate to a given temperature. Another technological operation used in the process of hardening metals is tempering, in which structural changes occur after heating to a low temperature with slow cooling. The ability to change the characteristics of steel through hardening is largely related to its original crystal structure and chemical composition, in which the most important components are carbon and alloying additives. They determine what the shape, size and configuration of the elements of the steel structure will be after its heat treatment.
What metals can be heated?
Metal hardening is a heat treatment that is most often applied to carbon and alloy steels in order to increase their hardness and improve strength characteristics. Heat treatment of non-ferrous metals, in particular tempering, annealing and hardening of copper, brass and bronze, as well as aluminum and titanium alloys, is somewhat less common. It should be noted that hardening of these compounds, unlike carbon steels, does not always lead to their strengthening; some copper alloys, on the contrary, then become more ductile and soft. Much more often, products made of non-ferrous metals are tempered to relieve stress after casting, stamping, rolling or drawing.
Introduction to Metals Technology
The steel we deal with most often is an alloy of iron and carbon.
The most widespread are structural steels of ordinary quality. They contain a small amount of carbon (less than 0.8%), so any attempts to harden products made from such material are doomed to failure. A small amount of carbon does not form grains of cementite (iron carbide, Fe₃C). It is this ingredient that is responsible for producing the hardness of hardened steel.
Checking the production of austenite when heated using a magnet:
When producing metal products, plastic materials are used. Using the pressing method, special original shapes are obtained. For example, car body parts are cold stamped using special dies. This tool consists of a matrix and a punch. The sheet blank is compressed between the components (die and punch) to obtain the final shape.
Read also: Homemade walk-behind tractor and attachments
The tool requires hardness, so during manufacturing, a material is chosen that can be hardened so that it does not deform in the future during use for stamping parts.
Hardened products from high-quality alloy steels:
In addition to carbon steels, in practice alloys with manganese, chromium, molybdenum, titanium and other elements are used. Their presence in the composition in a certain quantity is characterized by a certain brand. Components that improve the properties of the alloy are called alloying. They noticeably change the properties:
- Increases surface strength.
- Increases the hardness of parts at the blank stage.
- Can be hardened during hardening.
- Do not rust when exposed to aggressive environments.
Alloy steels are marked using their own method, in which certain alloy metals are designated by their own letters. The numbers after the letters indicate the content of a particular component in tenths of a percent. If it has only a letter, and no numbers follow it, then the ingredient can reach up to 1% in the alloy. For example, chronic hepatitis is characterized by the presence of:
- about 1% carbon;
- 0.8...1.2% chromium (X), this component gives stainless properties;
- 0.9...1.1% tungsten (B), this ingredient increases hardness and allows you to harden products;
- 0.8...1.4% manganese (G, that’s what the metallurgists agreed upon). Mn in steel imparts spring properties.
Properties of steel after hardening
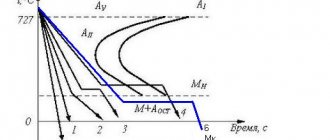
During the heating process, carbon steel goes through a series of phase changes in its structure, during which its composition changes, as well as the shape and elements of the crystal lattice. At a critical temperature of 723 °C, the decomposition of cementite (iron carbide) begins in the still solid metal and the formation of a uniform solution of carbon in iron, called austenite. This state of carbon steel is the initial state for hardening.
When cooled slowly, the austenite disintegrates and the metal returns to its original state. If the steel is cooled quickly, then the austenite does not have time to change, and at a certain cooling rate and threshold temperatures, crystal lattices and chemical compositions are formed, giving it different performance properties. This process is called hardening, and each type of it corresponds to a certain structure of already hardened steel, which has certain technical characteristics. The main phase states that are important during hardening are pearlite, sorbitol, troostite and martensite (see figure below).
The highest hardness is found in steel hardened to the state of martensite. In this way, the cutting tool is hardened, and the surfaces of parts exposed to friction during operation (bushings, cages, shafts, gears, etc.) are also hardened. After hardening with troostite, the steel becomes both hard and elastic. This type of heat treatment is applied to impact tools, as well as springs and spring shock absorbers. To obtain such properties of steel as wear resistance, elasticity and toughness, hardening to the sorbitol state is used. This heat treatment is used for rails and other structural elements operating under constant dynamic load. The listed phase states are characteristic of all carbon steels, but each grade is characterized by its own temperature ranges and cooling rates.
What is metal hardening?
One type of heat treatment is metal hardening. It consists of several stages performed in a certain sequence:
- Heating metal to a certain temperature. Dwell time for leveling over the entire depth of the part.
- Fast cooling.
- Tempering to relieve stress and correct hardness to a specified value.
During the manufacturing process, complex parts can undergo several different types of hardening.
Based on the depth of treatment, hardening is divided into two types:
- volumetric;
- superficial.
Basically, in mechanical engineering, volumetric heat treatment is used, when the part is heated to its entire depth. As a result of sudden cooling, after the completion of heat treatment, the hardness inside and outside differs by only a few units.
Surface hardening is used for parts that must be hard on top and ductile on the inside. The inductor heats the steel to a depth of 3–20 mm and immediately behind it there is a sprayer that pours water on the hot metal.
The steel is heated to austenite state. Each brand has its own temperature, determined from the table of the state of iron-carbon alloys. During sudden cooling, carbon remains inside the grain and does not enter the intercrystalline space. The transformation of the structure does not have time to occur, and the internal structure contains pearlite and ferrite. The grain becomes finer, the metal itself becomes harder.
What steels can be hardened?
When heated and rapidly cooled, internal changes in structure occur in all steels. Hardness increases only with carbon content greater than 0.4%. St. 35 according to GOST has it 0.32 - 0.4%, which means it can “get hot” - slightly change the hardness if the carbon is located at the upper limit.
Steels starting from CT45 and higher in carbon content are considered hardenable. At the same time, hardening of stainless steel with low carbon content, type 3X13, is possible. Chromium and some other alloying elements replace it in the crystal lattice and increase the hardenability of the metal.
High-alloy carbon steels contain substances that accelerate the cooling process and increase the steel's ability to harden. They require a complex step cooling system and high temperature tempering.
Temperature and heating rate
The heating temperature for hardening increases with the content of carbon and alloying substances in the steel. For St45 it is, for example, 630–650⁰, St 90HF - more than 800⁰.
High-carbon and high-alloy steels, when heated quickly, can “crack” - form small cracks on the surface and inside. They are heated in several stages. At temperatures of 300⁰ and 600⁰, exposure is done. In addition to equalizing the temperature throughout the depth, there is a structural change in the crystal lattice and a transition to other types of internal structure.
Properties of steel after hardening
After hardening of parts, structural changes occur that affect the technical characteristics of the metal:
- increases hardness and strength;
- grain decreases;
- flexibility and ductility decreases;
- fragility increases;
- abrasion resistance increases;
- fracture resistance decreases.
It is easy to obtain a high class of cleanliness on the surface of a hardened part. Raw steel is not polished, it drags on and on.
Classification of hot steel
Types of steel hardening are classified according to the type of heating source and method of cooling the metal. The main equipment for heating parts before hardening is still muffle furnaces, in which metal products of any size can be evenly heated. A high heating rate during continuous processing of products is ensured by hardening using high-frequency currents (induction hardening of steels) (see photo below). To harden the top layers of steel products, fairly inexpensive and effective gas-flame hardening is used, the main disadvantage of which is the inability to accurately set the heating depth. Laser hardening does not have these disadvantages, but its capabilities are limited by the low power of the radiation source. Methods for cooling a hardened part are usually classified according to the type of cooling medium, as well as sets and cycles of work operations. Some of them include tempering procedures, while others, such as various types of isothermal hardening, do not need it.
Hardening in one environment
With this method of hardening, a steel product heated to a given temperature is placed in a liquid, where it remains until it cools completely. Water is used as a quenching medium for carbon steels, and mineral oil is used for alloy steels. The disadvantage of this method is that after such hardening, significant stresses remain in the metal, so in some cases additional heat treatment (tempering) may be required.
Step hardening
Step hardening takes place in two stages. In the first step, the product is placed in an environment with a temperature several tens of degrees higher than the point at which martensite begins to appear. After the temperature is equalized throughout the entire volume of the metal, the part is slowly cooled, as a result of which a martensitic structure is uniformly formed in it.
Isothermal hardening
During isothermal hardening, the product is also kept in a hardening bath at a temperature above the martensite point, but slightly longer. As a result, austenite is transformed into bainite, a type of troostite. This steel combines increased strength with ductility and toughness. In addition, after isothermal hardening, the residual stresses in the product are reduced.
Hardening with self-tempering
This type of heat treatment is used to harden the impact tool, which must have a hard surface layer and a viscous middle. Its peculiarity is that the product is removed from the quenching container when it is not completely cooled. In this case, its internal part still contains enough heat to warm the entire volume of metal to the tempering temperature. Since reheating of the product is carried out without external influence due to internal thermal energy, this type of heat treatment is called quenching with self-tempering.
Light hardening
Light hardening is used for steel products whose surfaces should not be subject to oxidation during heat treatment. With this heat treatment, steel is heated in vacuum furnaces (see photo below) or in inert gas environments (nitrogen, argon, etc.), and cooled in non-oxidizing liquids or melts. This method is used to harden products that should not be subjected to further grinding, as well as parts that are critical to the carbon content in the surface layer.
Cooling steel during hardening
The basis of most coolants used in hardening steel products is water. It is important that such water does not contain impurities of salts and detergents, which can significantly affect the cooling rate. A container containing water for hardening metal products is not recommended for other purposes. It is also important to take into account that running water cannot be used to cool the metal during the hardening process. The optimal temperature for coolant is 30 degrees Celsius.
Hardening steel products using ordinary water to cool them has a number of significant disadvantages. The most important of them is cracking and warping of parts after they have cooled. As a rule, this cooling method is used when cementing metal, surface hardening of steel, or heat treatment of parts of a simple configuration that will later be subjected to finishing.
For products of complex shapes made from structural steels, another type of coolant is used - a 50% caustic soda solution heated to a temperature of 60 degrees Celsius. After cooling in such a solution, the hardened steel acquires a light shade.
It is very important to follow safety precautions when working with caustic soda; be sure to use a hood placed above the bathtub. When a hot part is lowered into a solution, vapors are formed that are very harmful to human health.
Hardening steel in a muffle furnace
The best coolant for thin-walled parts made of carbon steels and products made of alloys are mineral oils, which provide a constant (isothermal) cooling temperature, regardless of environmental conditions. The main thing to avoid when using such a technical fluid is getting water into it, which can lead to cracking of parts during their cooling. However, if water does get into such a coolant, it can be easily removed from it by heating the oil to a temperature above the boiling point of water.
Tempering steel using oil as a coolant has a number of significant disadvantages that you should definitely be aware of. When oil comes into contact with a hot part, vapors are released that are harmful to human health; in addition, the oil may catch fire at this moment. An oil bath also has the following property: after its use, a residue remains on the parts, and the coolant itself loses its effectiveness over time.
All these factors should be taken into account when hardening metals in an oil environment and the following safety measures should be taken:
- immerse parts in an oil bath using tongs with long handles;
- carry out all work wearing a special mask made of tempered glass and gloves made of thick fabric with fire-resistant properties or rough leather;
- reliably protect your shoulders, neck, chest with work clothes made of thick fire-resistant fabric.
Oil bath cooling
To harden certain grades of steel, cooling is carried out using an air flow created by a special compressor. It is very important that the cooling air is completely dry, as the moisture it contains can cause the metal surface to crack.
There are methods for hardening steel that use combined cooling. They are used to cool parts made of carbon steels that have a complex chemical composition. The essence of such hardening methods is that the heated part is first placed in water, where in a short time (a few seconds) its temperature drops to 200 degrees, further cooling of the part is carried out in an oil bath, where it should be moved very quickly.
Equipment for heat treatment of steels
The main equipment used for heat treatment of products made of steel and non-ferrous metals consists of two main groups: installations for heating workpieces and quenching baths. Heating devices include the following types of equipment:
- muffle thermal furnaces;
- induction heating devices;
- installations for heating in melts;
- gas plasma installations;
- laser hardening devices.
The first three types can heat the entire volume of the product to the required temperature, while the latter can only heat the surface layer of the metal. In addition, furnaces for hardening metals are produced and widely used, in which heating is carried out in a vacuum or in an inert gas environment.
VIEW Induction heater on AliExpress from 7,506 rubles → Quenching baths are represented by steel cooling tanks for various liquids, as well as special graphite crucibles and furnaces for molten salts or metals. Mineral oil, water and water-polymer mixtures are most often used as quenching liquids. Lead or tin are usually used for molten metals, and compounds of sodium, potassium and barium are used for molten salts. Quenching baths for liquid media have systems for heating and cooling the working fluid to the required temperature, as well as mixers for uniform distribution of the liquid and destruction of the steam jacket.
Coolants
The main coolant for steel is water. If you add a small amount of salts or soap to the water, the cooling rate will change. Therefore, under no circumstances should the quenching tank be used for other purposes (for example, washing hands). To achieve the same hardness on the hardened surface, it is necessary to maintain the coolant temperature at 20 - 30 degrees. You should not change the water in the tank frequently. It is absolutely unacceptable to cool the product in running water.
The disadvantage of water hardening is the formation of cracks and warping. Therefore, only products of simple shapes or cemented ones are hardened using this method.
- When hardening products of complex configurations made of structural steel, a fifty percent solution of caustic soda is used (cold or heated to 50 - 60 degrees). Parts heated in a salt bath and hardened in this solution turn out light. The solution temperature should not be allowed to exceed 60 degrees.
Modes
The vapors generated during quenching in a caustic solution are harmful to humans, so the quenching bath must be equipped with exhaust ventilation.
- Alloy steel is hardened in mineral oils. By the way, thin carbon steel products are also carried out in oil. The main advantage of oil baths is that the cooling rate does not depend on the oil temperature: at a temperature of 20 degrees and 150 degrees, the product will cool at the same rate.
Be careful not to let water get into the oil bath, as this may cause the product to crack. What is interesting: in oil heated to a temperature above 100 degrees, the ingress of water does not lead to the appearance of cracks in the metal.
The disadvantage of an oil bath is:
- release of harmful gases during hardening;
- formation of plaque on the product;
- oil's tendency to flammability;
- gradual deterioration of hardening ability.
- Steels with stable austenite (for example, X12M) can be cooled with air supplied by a compressor or fan. At the same time, it is important to prevent water from entering the air duct: this can lead to the formation of cracks in the product.
- Step hardening is performed in hot oil, molten alkalis, and low-melting salts.
- Intermittent hardening of steels in two cooling environments is used for processing complex parts made of carbon steels. First they are cooled in water to a temperature of 250 - 200 degrees, and then in oil. The product is kept in water for no more than 1 - 2 seconds for every 5 - 6 mm of thickness. If the exposure time in water is increased, cracks will inevitably appear on the product. Transferring the part from water to oil must be done very quickly.
Do you need to cut metal quickly and efficiently? Use a plasma cutter! How to do it correctly, read this article.
If you are interested in how to turn metal products, read the article at https://elsvarkin.ru/obrabotka-metalla/tokarnaya-obrabotka-metalla-obshhie-svedeniya/ link.
Temperature for hardening
The standard heating temperature of steel during quenching directly depends on the mass fraction of carbon and alloying additives. In general, the following relationship is observed: the lower the carbon content, the higher the quenching temperature. If the product is underheated, the required structure does not have time to form, and with significant overheating, decarburization, oxidation of the surface layer, changes in the shape and size of structural elements, as well as an increase in internal stress occur. The table below shows the quenching, annealing and tempering temperatures of some grades of carbon and alloy steels.
| steel grade | Temperature, C | ||
| hardening | annealing | vacations | |
| 15G | 800 | 780 | 200 |
| 65G | 815 | 790 | 400 |
| 15X, 20X | 800 | 870 | 400 |
| 30Х, 35Х | 850 | 880 | 450 |
| 40Х, 45Х | 840 | 860 | 400 |
| 50X | 830 | 830 | 400 |
| 50G2 | 805 | 830 | 200 |
| 40ХГ | 870 | 880 | 550 |
| OX13 | 1050 | 860 | 750 |
| 3Х13 | 1050 | 880 | 450 |
| 35ХГС | 870 | 860 | 500 |
| 30ХГСА | 900 | 860 | 210 |
| U7, U7A | 800 | 780 | 170 |
| P9, P12 | 1250 | 860 | 580 |
| R9F5, R9K5 | 1250 | 860 | 590 |
| R18F2 | 1300 | 900 | 590 |
| ШХ15 | 845 | 780 | 400 |
| 9ХС | 860 | 730 | 170 |
| R18K5F2 | 1280 | 860 | 580 |
| 1Х14Н18Б2БРГ | 1150 | 860 | 750 |
| 4Х14Н1482М | 1200 | 860 | 750 |
Determination of heating temperature in industrial production is carried out using contact and non-contact pyrometers. In recent decades, infrared devices have become widespread, making it possible to remotely measure the temperature at any point on the surface of a heated part. In addition, the approximate heating temperature of steel can be determined from color tables.
Performing hardening and tempering of steel parts at home
Heat treatment of metal products, including surface hardening of steel, not only increases the hardness and strength of the alloy, but also significantly increases internal stresses in its structure. To relieve these stresses, which can lead to breakage of the part during operation, it is necessary to release the steel product.
It should be borne in mind that such a technological operation leads to a slight decrease in the hardness of steel, but increases its ductility. To perform tempering, the essence of which is to gradually reduce the temperature of the heated part and maintain it at a certain temperature, furnaces, salt and oil baths are used.
Quenching and tempering steel at home
Temperatures at which tempering is carried out differ for different grades of steel. Thus, tempering of high-speed alloys is carried out at a temperature of 540 degrees Celsius, and for steels with a hardness of HRC 59-60, 150 degrees is sufficient. What is typical is that when high-speed alloys are tempered, their hardness even increases, and in the second case its level decreases, but the ductility index increases significantly.
Hardening and tempering of steel products, including stainless steel varieties, is quite acceptable (and, moreover, often practiced) at home, if the need arises. In such cases, electric stoves, ovens, and even hot sand can be used to heat steel products. The temperatures to which steel products should be heated in such cases can be selected using special tables. Before hardening or tempering steel products, they must be thoroughly cleaned; their surface should be free of dirt, traces of oil and rust.
After cleaning, the steel product should be heated so that it becomes evenly red-hot. In order to heat it to such a state, it is necessary to perform heating in several approaches. After the required state has been achieved, the heated product should be cooled in oil and then immediately placed in an oven preheated to 200 degrees Celsius. Then you need to gradually reduce the temperature in the oven, bringing it to 80 degrees Celsius.
This process usually takes an hour. Further cooling should be carried out in the open air, with the only exception being products made of chromium-nickel steels, for which oil baths are used to reduce the temperature. This is due to the fact that steel of such grades, when cooled slowly, can acquire so-called temper brittleness.
Many tools that you work with in a workshop must have a certain strength. Home craftsmen ask the question: “How to harden steel at home?” The quality of the product often depends on the hardness of the cutting edge. Of course, many people know that hardening requires heating an object and then cooling it sharply. At the same time, you can hear how someone achieved extraordinary hardness and strength of any part in a similar way.
Attempting to harden the product yourself on a gas stove or other heat source is unsuccessful. A workpiece heated to a red glow with sudden cooling increases the strength slightly, and sometimes the opposite phenomenon occurs - the hardness decreases.
Metal heating technology
Steel hardening technology requires compliance with a number of requirements for the heating and cooling processes of hardened parts.
First of all, this relates to the rate of heating and cooling of the metal. The economic indicators of the thermal process require the fastest possible increase in temperature to the nominal temperature, since this consumes less energy. However, rapid heating leads to a large temperature difference between the surface layer and the core of the product, which can lead to its deformation and the occurrence of cracks. Therefore, heating to the entire depth of the part until it is completely heated must proceed smoothly, and its time is determined by a thermal technologist using empirical formulas and tabular values. The process of formation of the structure and composition of the hardened metal directly depends on the rate and temperature parameters of cooling of steel heated above the critical point. For example, when rapidly cooled in water at room temperature, carbon steel with a martensitic structure can be obtained, and when cooled in oil or hot water, troostite is obtained. Each grade of steel has its own characteristics and hardening temperature conditions, which, among other things, depend on the size and shape of the part. Therefore, in production, heat treatment of parts is carried out in accordance with route technology and operational cards developed for each product.
What is the hardening of metal?
The ancient blacksmiths knew why steel was hardened for. The correctly selected steel hardening temperature allows you to change the basic operational characteristics of the material, as the structure is transformed.
Hardening is the heat treatment of steel, which today is carried out to improve the mechanical properties of the metal. The process is based on the rearrangement of the atomic lattice due to exposure to high temperature followed by cooling.
Steel hardening technology makes it possible to give inexpensive grades of metal higher performance qualities. Due to this, the cost of manufactured products is reduced and the profitability of established production is increased.
The main goals pursued during hardening:
- Increasing the hardness of the surface layer.
- Increase in strength index.
- Reducing ductility to the required value, which significantly increases bending resistance.
- Reducing the weight of products while maintaining strength and hardness
There are a variety of methods for hardening steel followed by tempering, which differ significantly from each other. The most important heating modes are:
- Heating temperature.
- Time required for heating.
- The holding time of the metal at a given temperature.
- Cooling rate.
Changes in the properties of steel during hardening can take place depending on all of the above indicators, but the most significant is the heating temperature. How the restructuring of the atomic lattice will occur depends on it. For example, the holding time when hardening steel is selected in accordance with the strength and hardness the gear must have to ensure long-term operation under conditions of increased wear.
Steel hardening colors
When considering which steels are subject to hardening, it is worth considering that the heating temperature depends on the level of carbon content and various impurities. The units of steel hardening are represented by the maximum temperature as well as the holding time.
When considering this process of changing basic performance properties, the following points should be taken into account:
- Hardening is aimed at increasing hardness. However, as hardness increases, the metal becomes more brittle.
- A layer of scale may form on the surface, since the loss of carbon and other impurities in the surface layers is greater than in the middle. The thickness of this layer is taken into account when calculating the allowance and the maximum dimensions of future parts.
Carbon steel is hardened taking into account the rate at which cooling will occur. If the developed technologies are not followed, a situation may arise when the rearranged atomic lattice goes into an intermediate state. This will significantly deteriorate the basic qualities of the material. For example, cooling at too high a rate causes the formation of cracks and various defects that prevent the workpiece from being used in the future.
The steel hardening process involves the use of chamber furnaces, which can heat the environment to a temperature of 800 degrees Celsius and maintain it for a long period. This allows you to extend the steel hardening time and improve the quality of the resulting workpieces. Some steels are suitable for hardening only if the environment is heated to a temperature of 1300 degrees Celsius, for which other furnaces are installed.
A separate technology is being developed for the case where the workpiece has thin walls and edges. It is represented by gradual heating.
Full hardening is usually used for steels and parts that are not subject to cracking or warping.
Often, staged heating technology involves reaching a temperature of 500 degrees Celsius in the first stage, after which a certain period of time is maintained to ensure uniform heating and the temperature is raised to a critical value. Cold hardening of steel does not lead to the restructuring of the entire atomic network, which determines only an insignificant increase in operational characteristics.
As previously noted, there are different types of hardening of steel, but it is always necessary to ensure uniform heating. Otherwise, the restructuring of the atomic lattice will proceed in such a way that serious defects may appear.
Cooling methods
By cooling steel to different temperatures and at different speeds, it is possible to obtain different structures of its crystal lattice with elements of different sizes and shapes. The combination of these characteristics with the chemical composition determines its performance qualities such as hardness, fragility, viscosity, strength, elasticity, etc. Therefore, there are many cooling technologies and their varieties, among which the following technological groups can be distinguished:
- Cooling in one component. The product is immersed in liquid and remains in it until it cools completely.
- Intermittent hardening in two coolers. The product is first placed in a fast-cooling liquid, and after reaching a given temperature, it is transferred to a slow-cooling environment.
- Jet cooling. The heated part is intensively irrigated with a coolant flow (see photo below).
- Airflow The surface of the product is blown with a stream of air or inert gas.
In the practical application of hardening, all these types of cooling can have different variations or be combined with each other.
Cooling media
Water is usually used as a coolant when hardening carbon steels: both pure and in the form of aqueous solutions (salt and alkaline). Alloy steels require a lower cooling rate, so mineral oils and air are used for them. During stepwise and isothermal hardening, the cooling medium is molten salts, alkalis and metals. In some types of hardening, the cooling media alternate to obtain the required steel structure.
| № | Structure | Cooling medium | Hardness (HBW) |
| 1 | Martensite | Cold water | 500÷750 |
| 2 | Troostitis | Oil | 350÷500 |
| 3 | Sorbitol | Air | 250÷350 |
| 4 | Perlite | As the oven cools down | 150÷250 |
The influence of cooling speed on the final result
When hardening steel, cooling must occur at a rate that prevents the decomposition of austenite into ferrite and iron carbide, which begins to occur at temperatures below 650 °C. Further reduction in temperature should be carried out more slowly, since such a speed ensures a decrease in the internal stresses of the steel. Rapid and complete cooling in cold water produces martensite, which has maximum hardness but is quite brittle. With a rapid decrease in temperature by 200÷300 °C, the decomposition of austenite stops, and further slower cooling forms phase states in the steel with lower hardness, but with increased strength and wear resistance. The cooling rate is controlled by the type of quenching medium used and its temperature (see table below).
| № | Cooling medium | Cooling rate (deg/sec) |
| 1 | Air | 5 |
| 2 | Mineral oil | 150 |
| 3 | Water at room t° | 700 |
| 4 | Water at 80 °C | 1400 |
| 5 | 10% sodium chloride solution | 2100 |
| 6 | 10% sodium hydroxide solution | 1600 |
What does proper hardening of steel improve?
If you ask the average person who has nothing to do with knife forging, the question “What does hardening give?” he will first talk about strength. In general, he will be right, although of the several qualities that hardening improves, hardness will still be the leader. But first things first.
- The hardness of blade steels is typically measured using the Rockwell Hardness Scale (HRC); European knives barely reach 60 HRC, Asian knives slightly exceed this mark. If we scratch two identical alloys of different hardness against each other, marks will remain on the softer one; Thus, hardness gives us an idea of how well an alloy resists mechanical damage.
- Strength usually means steel’s resistance to destruction (bending, impact, etc.) - for a knife this is important when, for example, we test it “for bending”. If the steel is damp, the blade will remain partially deformed after bending. True, if the steel is overheated, it will be even worse - the blade will break; Therefore, when hardening, it is important to maintain a golden mean.
- Elasticity. This is exactly what we talked about a little higher - the ability to return to its original shape after removing the load. If the hardening is done according to all the rules, everything will be fine with this indicator: when bent by about 10 degrees (and for thin kitchen knives up to 30), the blade will return to its original shape.
- Wear resistance. The correct hardening regime improves all the indicators that are included in this concept: the ability to resist mechanical and abrasive wear, the ability to hold an edge and resistance to shock loads.
The main thing in the pursuit of all these qualities is to achieve by hardening such a compromise of all the above properties so that the knife cuts well and is durable.
Differences between hardenability and hardenability
Each grade of steel has a certain hardenability, which is characterized by its ability to acquire the required hardness during hardening. The main factors affecting the hardenability of steel are the percentages of carbon and alloying additives. The lower limit of carbon content, after which steel does not accept hardening, is 0.2%. Hardenability is characterized by the depth of penetration into the volume of metal of a hardened structure (fully martensitic or consisting of troostite and martensite). Alloying additives in the form of molybdenum, chromium, nickel, etc. increase both hardenability and hardenability, and the addition of cobalt reduces them.
Methods of surface hardening of steel
There are 4 main methods of surface hardening: with induction heating using high-frequency current, with electric contact heating, with heating using gas flame burners and hardening using an electrolytic solution. For processing small parts, the latter method is often used.
For medium-sized products, the first two are used, and for large-sized elements, heating using gas burners is best suited. For cooling, the same liquids are used as for complete hardening. In rare cases, gas refrigeration units are used for particularly large structures. And the last type of processing is called incomplete hardening of steel. It involves slow cooling of the heated material, as a result of which some of the carbon atoms have time to leave the molecular network of iron and return to their normal state.
Thus, a partially hardened metal is formed. This type of processing is used when it is necessary to specifically leave weak points in the structure of the material. This approach is used in the automotive industry to create what is known as controlled crash deformation. It is designed specifically to reduce passenger injuries and fatalities.
Defects during hardening of steel
The cause of defects during steel hardening is a number of physical and chemical factors that arise when there is a deviation from the specified parameters of the thermal process or due to the heterogeneity of the workpiece being hardened. Uneven heating or cooling of the product can lead to its deformation and internal cracks. The same reason can cause uneven phase transformations in different parts of the product, as a result of which the metal will have a structure that is heterogeneous in composition and hardness. Burnout of steel occurs due to the penetration of oxygen into the surface layer of the metal, which leads to the formation of oxides that separate its structural elements and change the physical properties of the surface layer. The reason for decarburization during steel hardening is the burnout of carbon when excess oxygen enters the furnace. These types of defects are irreparable, and the only way to deal with them is to check the tightness of the furnace or hardening in a vacuum and inert gases.
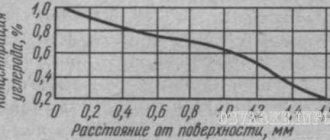
Scales and a critical decrease in carbon concentration during heating
Even a small concentration of oxygen in a hardening furnace leads to the appearance of surface scale, which is a consequence of the oxidation of the metal during its heat treatment. The same reason can cause a decrease in the amount of carbon in the surface layer of the workpiece. It is possible to completely get rid of such phenomena only by using vacuum furnaces, which provide so-called light hardening, as well as by heating the product in a nitrogen or argon environment. To minimize oxidation and decarburization, the hardening furnace must be as sealed as possible, which to some extent limits the flow of oxygen into its working space.
For hardening metals, it is recommended to use transformer or industrial oil I-20. It is not easy for a private owner to get it, so I would like to hear in the comments to this article your opinion on the possibility of using used car oil or other automobile oil for hardening steel.
Hardening technology
Muffle furnace for heating parts:
To understand how to properly harden steel, you need to look at the process. It is as follows:
- The workpiece is heated to a temperature of 750…770 ⁰С. In this state, it stops being magnetic. Metallurgists call this mode austenitic. High plasticity occurs. The metal grains become large and the bond between them is destroyed.
- It takes time for the entire part to warm up. Steels have a peculiarity: only the surface layer can be heated, just a few fractions of a millimeter. Below this layer the temperature can be 30...50 ⁰C lower. If you do not heat the metal through its thickness, then only surface hardening will occur. When tested on a Rockwell device, the hardened layer is punctured; hardness is not guaranteed.
- The heated metal is hardened in water or oil. The choice of quenching medium is explained by the fact that when quenching in water, water vapor forms around the metal. It reduces the cooling rate several times. Steam can have a temperature of up to 200...250 ⁰С, so there is no real hardening. When hardening in an oil environment (its boiling point is 350...380 ⁰C), the cooling rate is several times higher. Experienced craftsmen do not simply lower the object into oil one time, but perform several successive liftings and lowerings up and down. This is achieved by the interaction of the metal with new portions of oil, the cooling rate increases.
- During hardening, large grains obtained by heating to austenite turn into small grains (the size decreases thousands of times). It is the sharp decrease in the grain structure that contributes to an increase in surface hardness.
- When hardening, internal stresses arise inside the metal. Sometimes you can observe how thin parts burst under light load. It is necessary to eliminate them by briefly heating to the tempering temperature.
- In practice, holidays are divided into several modes. The most common is low tempering, which occurs at a temperature of 200...220 ⁰С. At home, it can be done in the oven of a regular gas stove. It is heated to a given temperature, parts that need to be partially released are placed. Then the metal is allowed to cool along with the entire plate (about 1...2 hours).
- Parts with low tempering last several times longer than hardened ones, but without subsequent tempering.
Oil hardening:
Characteristics of steel: hardenability and hardenability
The important characteristics of steel - hardenability and hardenability - should not be confused.
Hardenability
This characteristic indicates the ability of steel to gain hardness after hardening. There are types of steel that are difficult to harden and after the heat treatment process the steel becomes insufficiently hard. They say about such material that it “did not accept hardening.”
The hardness of martensite is related to the degree of distortion of its crystal lattice. A lower carbon content in martensite contributes to less distortion in the crystal lattice, which means the hardness of the steel will be lower. If the steel contains less than 0.3% carbon, then the hardenability of such an alloy is low, and usually such alloys are not hardened.
Hardenability
This characteristic can indicate how deeply the steel has been hardened. When hardening, the surface of a steel part cools faster than the core . This occurs because the surface is in direct contact with the cooling fluid, which removes heat. And the central part of the steel part gives off its heat through the thickness of the metal and the surface, where it is absorbed by the coolant.
Hardenability is affected by the critical hardening rate - the lower it (speed), the deeper the steel is hardened. For example, coarse-grained steel, which has a low critical hardening rate, is annealed deeper than fine-grained steel, which has a high critical hardening rate.
The depth of hardenability depends on the initial structure of the alloy being hardened, the heating temperature and the quenching medium. The hardenability of steel is determined by fracture, microstructure and hardness.
Why is steel hardening needed and how is it carried out?
Hardening is a type of heat treatment of metals that involves heating above a critical temperature followed by rapid cooling (usually) in liquid media. Critical is the temperature at which a change in the type of crystal lattice occurs, that is, a polymorphic transformation occurs. It is determined by the iron-carbon diagram. photo