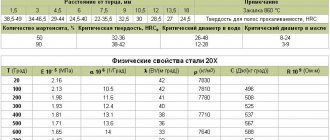
Welding mode
Joining high-alloy steels and alloys requires the correct setting of the welding mode. The quality of the finished seam largely depends on this. We recommend setting a low welding current and creating narrow seams. This can be achieved using welding wire or electrodes with a diameter of 2-3 millimeters. We also recommend reducing the electrode extension by 2 times more than usual. This will make your welding easier. After all, welding high-alloy steel is largely difficult due to high electrical resistance and reduced electrical conductivity. And by reducing the electrode overhang, you eliminate these shortcomings.
Welding of chromium steels.
Chromium stainless steels contain about ten percent chromium and 0.1-0.4% carbon. Such steels weld quite well with a carbon content of no more than 0.2%. To protect against burnout when welding chrome-plated steel, it is necessary to use a protective coating for the assembled surface, as well as use alloyed additives or electrodes containing chromium. It is better to weld such steel using an electric arc with preheating of the metal before welding to 300 degrees and subsequent annealing of the seams after welding to 800 degrees to restore the toughness of the metal. It should be taken into account that the higher the content of alloyed chromium, the higher the temperature of subsequent heat treatment should be.
Chromium-nickel steels are susceptible to any welding method. But it should be taken into account that at high temperatures chromium carbides precipitate, which leads to a loss of anti-corrosion properties at welding joints. To maximize the resistance of such metal structures to corrosion during welding, titanium or niobium should be added. Subsequent annealing after welding and hardening of the seams by sudden cooling also contribute to maintaining corrosion resistance.
Selecting Electrodes
When welding high-alloy steels, it is recommended to use electrodes with a basic coating, which should contain protective and alloying elements. The rod itself must also be highly alloyed. Electrodes and metal of similar composition will work in conjunction with each other, forming a high-quality seam.
If you need to weld high-alloy austenitic steel, we recommend using TsT-15 electrodes. They are excellent for steel grade E-08Х19Н10Г2Б, since they contain up to 5% ferrite phase. And this is very good.
High alloy steels
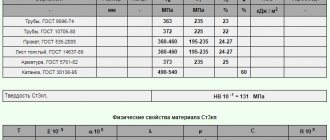
High-alloy steels have a higher content of alloying elements - Cr and Ni (usually not lower than 16% and 7%, respectively). They give such metals the appropriate structure and necessary properties. Highly alloyed steels, compared to less alloyed steels, have high cold resistance, corrosion resistance, heat resistance and heat resistance. Despite the high properties of these steels, their main service purpose is determined by the appropriate selection of alloying composition. Accordingly, they can be divided into three groups: heat-resistant, heat-resistant and corrosion-resistant.
After appropriate heat treatment, high-alloy steels have high strength and plastic properties. Unlike carbon materials, when hardened, these materials acquire increased plastic properties.
The structures of high-alloy steels are very diverse and depend mainly on their chemical composition, that is, on the content of the main elements: chromium (ferritizer) and nickel (austenitizer). The structure is also affected by the content of other alloying elements, ferritizers (Mo, Ti, Si, Al, W, V) and austenizers (Co, Cu, C, B).
Selection of fluxes
Welding of alloy and carbon metals can also be performed using flux. But here, as in the case of electrodes, you need to use special fluxes and correctly combine them with the welding wire. The fluxes themselves must be fluoride, and the wire must be highly alloyed, like the metal. We recommend ANF-5 flux; it copes well with its protective function and improves the quality of the seam when welding high-alloy steel.
Thanks to the use of ANF-5 flux, the seam will not be susceptible to the formation of pores, cracks and other weld defects. For this reason, such a flux is often used not only in home welding, but also in large-scale welding production. By the way, you can use other oxide-based fluxes. Their properties will not differ much from ANF-5.
As an alternative, we suggest using AN-26 flux. It is also made on the basis of oxides and contains little silicon, so the seam will be formed efficiently and quickly. But please note that there is a high probability of strong oxidation of titanium and aluminum, and even a well-chosen wire will not help; silicon will actively transfer into the seam. Because of this, hot cracks and pores may likely appear, and in general the seam will be fragile. So use this flux on less critical objects.
Also pay attention to flux brand AN-292. It is made on the basis of highly stable oxides and has proven itself in operation. But you need to monitor the amount of hydrogen; if there is too much of it, the seam may turn out to be porous after welding is completed.
Medium alloy steels
Such alloys are characterized by more than twice the carbon content. Ni, Mo, Cr, V, W are most often used as additives. Ideal metal characteristics are achieved by hardening and low tempering. These types of steels are thoroughly cleaned from various types of non-metallic impurities. To achieve optimal properties, remelting and thermomechanical processing are used.
For reliability and wear resistance of welds, it is necessary to obtain ideal chemical properties of the joints. Welding materials must contain a smaller volume of alloying elements than the base metal. With the help of properly selected material, you can obtain excellent strength and other weld qualities when welding alloy steels.
Medium alloy alloys with high strength and hardenability levels must be welded using materials that will give the joints maximum deformability. For such purposes, low-alloy electrodes that do not contain organic substances and are calcined at high temperatures are used. When welding, it is necessary to ensure optimal working conditions - to prevent the presence of moisture and the appearance of rust in the weld pool, so as not to increase the level of hydrogen.
The optimal method for alloy and carbon steels is argon welding with non-consumable electrodes. This type is optimal for mechanized penetration, providing optimal depth and uniformity of the process.
Gas welding of alloy steels is carried out with acetylene and oxygen, which provides a high-quality weld. Substitute gases should not be used in this case. However, even acetylene and oxygen do not fully guarantee a high-quality seam. This can only be achieved by using arc welding.
Shielding Gas Selection
Shielding gas can also be used. Helium, argon and carbon dioxide are often used. And in some cases, a mixture of these gases is used. The technology for welding high-alloy steels using shielding gases has proven itself well. But in addition to gas, you will need to purchase more electrodes. We recommend non-consumable tungsten. Welding must be carried out using direct current with reverse polarity. If the steel contains a lot of aluminum, then you can cook with direct polarity to quickly destroy the oxide film that interferes with the formation of the weld.
Sometimes when welding austenitic steels using shielding gases, unstable arc combustion is observed. To correct this problem, you can mix argon and oxygen or argon and carbon dioxide. This way the arc will burn stably and the seam will not be porous.
As for carbon dioxide, it has many positive properties. Thanks to it, the likelihood of pore formation is minimal. And in combination with argon, carbon dioxide shows the best results. So if you have the opportunity to use a mixture of these two gases, then be sure to try it in your practice.
But there is also a drawback. When welding in carbon dioxide, the metal spatters much more, and this worsens the anti-corrosion properties of steel. And the technology of welding alloy steels using carbon dioxide is associated with another problem - the active formation of an oxide film on the metal surface, which is difficult to remove. And if with single-layer welding this drawback is not so significant, then with multi-layer welding the oxide film simply does not allow the seam to form.
In general, the use of shielding gases when welding high-carbon steels has proven to be quite effective. There is no need to worry about selecting electrodes and their coatings, and there is no need to select the flux composition. After all, the gas perfectly protects the weld pool and allows you to form a high-quality, durable seam. If, of course, you follow welding technology.
Features of welding alloy steels
Based on the properties of alloy steels , it is most rational to use a neutral protective medium or protective alloying fluxes in combination with alloyed wire. However, in this case, the likelihood of defect formation due to hydrogen increases.
To prevent the formation of these defects when welding low-alloy and medium-alloy steels, as well as some high-alloy steels (pure austenitic steels, high-chromium martensitic steels), it is advisable to use an oxidizing protective medium in combination with alloyed wire. When welding purely austenitic steels, the creation of oxidizing conditions in the welding zone makes it possible to reduce the content of not only hydrogen, but also silicon and thereby increase resistance to the formation of hot cracks.
If there are significant amounts of manganese in the weld, creating oxidizing conditions in the welding zone is irrational, since in this case contamination of the weld metal with oxide inclusions and intense oxidation of manganese is observed.
For welding low-alloy and medium-alloy steels, the composition of the weld metal is chosen to be close to the composition of the base metal. However, given that carbon increases the likelihood of the formation of both cold and hot cracks, usually when welding low-alloy steels, the carbon content in the weld is set to no more than 0.15%, and when welding medium-alloy steels 0.23%. In this case, the necessary properties of the weld metal are obtained through additional alloying. In the case of welding heat-resistant steels, it must be taken into account that, as a rule, welded joints are subjected to long-term operation at elevated temperatures.
Under such conditions, diffusion processes develop significantly. If there is a difference in the composition of the weld metal and the base metal, especially in carbide-forming elements, a redistribution of carbon, which has increased diffusion mobility compared to other steel components, is possible. This can lead to the formation in the region of the fusion boundary of diffusion layers that are decarbonized on the side of the metal, which has a reduced content of carbide-forming elements, and with an increased carbon content on the side of the metal, which has a high content of carbide-forming elements. To prevent the development of these processes, the composition of the weld metal must be close to the base one. First of all, this relates to the content of carbide-forming elements.
In order to prevent the formation of crystallization cracks when welding heat-resistant steels, the carbon content in the weld metal is limited to 0.07–0.12%, and the necessary properties of the weld metal are ensured by the additional introduction of alloying elements that do not cause an increase in the development of diffusion redistribution of carbon between the base metal and weld metal with the formation of interlayers. For this purpose, it is rational to use complex alloying of the weld metal with chromium, molybdenum, vanadium, and tungsten so that the concentration gradient for each element in the fusion zone is small.
In relation to high-alloy steels, the choice of filler metal is differentiated. For welded joints operating at high temperatures, the most optimal composition of the weld metal is a composition corresponding to the composition of the base metal.
To improve the properties of the weld metal, especially when welding single-phase steels - high-chromium, chromium-nickel austenitic, etc., it is rational to introduce small additions of elements into the weld pool metal that provide structure refinement, for example titanium.
When choosing the composition of the weld metal, it is necessary to take into account the tendency of high-alloy steels to form hot cracks and provide measures to prevent them. Depending on the stability of austenite, various methods of combating hot cracks are used. When welding steels with metastable austenite (type 18-9), the formation of hot cracks is prevented by forming the weld metal with a two-phase austenitic-ferritic structure. With an increase in the amount of ferrite phase, the resistance of the weld metal to the formation of hot cracks increases (Fig. 7.9). Austenitic-ferritic welds acquire the greatest resistance to the formation of hot cracks when the ferrite phase content is in the range of 20-60%.
| Rice. 7.9. The influence of the amount of ferrite phase on the critical strain rate and the formation of hot cracks in ferritic-austenitic chromium-nickel welds containing 20-22% Cr. |
The presence of ferrite in austenitic steel increases the likelihood of the formation of a brittle σ-phase during prolonged operation at high temperatures, therefore, in most cases, the amount of ferrite in the weld metal is limited to 2-7%.
If welded joints operate at temperatures below 300 °C, increasing the amount of ferrite phase is rational, since the presence of a ferrite phase increases the corrosion resistance of the weld metal.
Chemical analysis of the weld metal, providing a given structure, is determined using a structural diagram (Fig. 7.10) based on equivalent concentrations of chromium and nickel. The equivalent content of chromium and nickel in the weld metal is determined by the formulas
Nieq = % Ni + 30% C + 30% N + 0.5% Mn; C-eq = % Cr + 2% Mo + 1.5% Si + 5% Ti + 2% Nb + + 2% Al + 1.5% W + % V.
| Rice. 7.10. Structural diagram of welds (according to Scheffler) |
The content of elements in the weld metal is calculated using formula (2.3) taking into account the transition coefficients. Since the formation of the structure largely depends on the crystallization conditions, and the structure diagram was developed in relation to manual arc welding, in most cases the assessment from the structure diagram is only qualitative.
When welding high-chromium steels of the martensitic class, the formation of a ferrite component in the structure of the weld metal in order to reduce the likelihood of hot cracks is not used, since in this case structurally free low-carbon δ-ferrite appears in the weld metal, which does not undergo transformations. As a result of the formation of a heterogeneous structure, the impact strength of the weld metal decreases and its susceptibility to the formation of cold cracks increases.
When welding high-chromium ferritic steels, the formation of welds with a two-phase austenitic-ferritic structure is rational, since it makes it possible to increase their resistance to the formation of hot cracks.
When welding deep austenitic steels, the two-phase structure of the weld metal is not used to prevent the formation of hot cracks, since this can dramatically change the performance properties of the weld metal compared to the base metal. When welding deep austenitic steels, the formation of hot cracks in the weld metal is prevented by using filler materials with a low content of harmful impurities (sulfur and phosphorus), as well as other elements that increase the likelihood of hot cracks. The likelihood of hot cracking in austenitic welds increases in the presence of silicon. The harmful effects of silicon are associated with the fact that silicon contributes to the development of chemical heterogeneity. Carbon neutralizes the harmful effects of silicon and reduces the likelihood of hot cracks forming in austenitic welds.
As a rule, cracks do not form in purely austenitic (ferrite-free) welds if / Si/C≤5. The nature of this combined action of silicon and carbon has not yet been sufficiently studied.
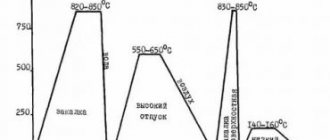
The resistance to the formation of hot cracks in austenitic welds increases when they are alloyed with molybdenum and manganese (up to 5-7%). In addition to changing the composition of the weld metal when welding purely austenitic steels, to prevent the formation of hot cracks, modes with low heat input are used (Fig. 7.11), as well as modes that ensure the formation of welds with a high aspect ratio.
Pore formation during welding of alloy steels is mainly due to hydrogen and nitrogen. The likelihood of pore formation due to the release of carbon monoxide is small, since the weld pool, as a rule, provides a sufficient concentration of strong deoxidizers (for example, silicon). To prevent the formation of pores due to hydrogen, various techniques are used to prevent hydrogen from entering the weld pool. Nitrogen-related porosity is prevented by providing good protection of the weld pool metal from the air atmosphere.
| Rice. 7.11. The influence of welding heat input and silicon content in the weld metal of type 0Х23Н28МЗДЗТ on the formation of hot cracks during automatic welding under AN-18 submerged arc (highly oxidizing low-silicon), thickness of the welded metal ≤16 mm: O - no cracks; X - cracks in the crater; ● — cracks along the entire length of the seam |
Welding manganese steel.
Welding manganese steel also has its own characteristics. To prevent the formation of cracks in the seams, welding should be carried out using electrodes made of manganese-nickel steel with a special coating or electrodes of the same chemical composition as the metal being welded. Welding should take as short a time as possible. The area to be welded must be cooled immediately to reduce the thermal effect on the welds being produced.
The preparation of parts for welding must be carried out in strict accordance with the planned drawings. Edge cutting must be done before hardening the parts to be joined. Poor preparation can lead to lack of penetration and cracks. To obtain high-quality seams, the edges of the surfaces being welded must be thoroughly cleaned of scale, slag, grease, and moisture. The surface should be cleaned to a width of 10 cm from the edge.
The assembly of steel products made from alloy steel should be approached with special care. Tack welding of the structure to be welded must be made with gaps of a certain size. Typically, tack welds should not exceed three times the thickness of the steel sheets being welded in length and reach no more than 0.7 times the thickness of the sheet in height.
How do alloying impurities affect weldability?
As previously noted, the inclusion of a large number of alloying elements leads to a change in the main characteristics. Let us note the following points:
- With a low concentration, steel is better weldable.
- Some chemicals can increase the indicator in question, while others can worsen it.
That is why when choosing an alloy alloy, attention is paid not only to the type of alloying elements, but also to their concentration. Accepted GOST standards determine that the labeling may indicate the main chemical substances and their quantities in the composition.
Austenitic steels: composition and properties
Austenitic steel is a metal to which chromium and nickel have been added in percentages of 18% and 10% respectively. Because of this, they are also known by the digital abbreviation 18-10.
The main advantage of this class of steel is corrosion resistance due to the addition of chromium. The presence of a chromium additive in an amount of 18% makes the steel resistant to many oxidizing environments (for example, nitric acid).
Adding 9-12% nickel to steel transforms the material into the austenitic class. This process increases the usability of steel, namely increases ductility and reduces the likelihood of grain appearing.
Specific properties:
- heat resistance;
- heat resistance;
- cryogenicity;
- corrosion resistance.
Instead of chromium and nickel, austenitic steel may contain other additives: ferritizers and austenizers.
Welding technologies
To minimize the occurrence of defects in the further operation of chromium-nickel steels, it is necessary to correctly select the optimal method for welding austenitic steel.
The main methods of welding austenitic steel:
- manual arc;
- electroslag;
- in an atmosphere of protective gases.
Manual arc welding
Manual arc welding is a fairly maneuverable method. This welding occurs in such a way that the chemical composition remains unchanged at different spatial positions and possible positions of the joints.
It is important to calculate the size of the deposited metal and the degree of penetration of the base metal layer. It is possible to fulfill these conditions by changing the composition of the coating of the electrodes used. The coating is selected so that in the end there are no hot cracks in the weld and primary ferrite is present in the required amount. For this purpose, electrodes containing fluorine and calcium are often used.
Optimal recommendations for manual arc welding:
- thread seams using electrodes with a cross section of 3 millimeters;
- Calcinate the welding electrodes for 60-90 minutes at a temperature from 250 oC to 400 oC (this must be done before starting welding). This prevents the formation of pores in the connecting seam.
Suitable electrodes are used with direct current and always with reverse polarity. At maximum current, welding is performed in the bottom position. And if work is required in a vertical or ceiling position, you need to take 10-30% less current.
Electroslag welding
The technology of performing electroslag welding work itself minimizes the possibility of hot cracks forming.
Advantages of this welding technique:
- No significant deformations in the corner and butt areas.
- Slow speed of movement of heating equipment.
- Soft crystallization of the weld pool.
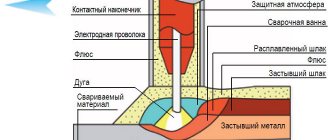
Electroslag welding scheme
For this type of welding, electrodes in the form of plates with a thickness of 6 to 20 mm or wire with a thickness of 3 mm are used.