Types of crystal lattices
All metals in the solid state are crystals.
A crystal is a collection of atoms located in space not randomly, but in a geometrically correct sequence. The spatial arrangement of atoms forms a crystal lattice. Positively charged ions are correctly located at the nodes of the spatial crystal lattice of the metal, and free electrons—electron gas—move between them. Moving from one cation to another, they bond between the ions and transform the metal crystal into a single whole. This bond, called a metallic bond, occurs between metal atoms due to the overlapping electron clouds of outer electrons. A metallic bond differs from a non-polar covalent bond in that it is non-directional. In a metal-type crystal, electrons are not fixed between two atoms, but belong to all atoms of a given crystal, that is, they are delocalized. Features of the structure of metal crystals include large coordination numbers – 8÷12, which correspond to a high packing density.
The crystal lattice of each metal consists of positively charged ions of the same size, located in the crystal according to the principle of the most dense packing of balls of the same diameter.
There are three main types of packaging, or crystal lattice.
1. Body-centered cubic lattice with a coordination number of 8 (sodium, potassium, barium). The metal atoms are located at the vertices of the cube, and one is in the center of the volume. The packing density of spherical ions in this case is 68%.
2. Face-centered cubic lattice with a coordination number of 12 (aluminum, copper, silver). Metal atoms are located at the vertices of the cube and at the center of each face. Packing density – 74%.
3. Hexagonal lattice with coordination number 12 (magnesium, zinc, cadmium). Metal atoms are located at the vertices and center of the hexagonal bases of the prism, and three more are located in its middle plane. Packing density – 74%.
Due to the unequal density of atoms in different directions of the crystal, different properties are observed. This phenomenon, called anisotropy, is characteristic of single crystals - single crystals. However, most metals under normal conditions have a polycrystalline structure, that is, they consist of a significant number of crystals, or grains, each of which is anisotropic. Different orientations of individual grains lead to averaging of the properties of a polycrystalline metal.
The characteristics of crystal lattices determine the characteristic physical properties of metals.
Properties of alloys
The properties possessed by metal alloys are divided into:
- Structurally insensitive. They are determined by the properties of the components and their percentage. These include :
- density;
- melting temperature;
- thermal and elastic characteristics;
- coefficient of thermal expansion;
- structurally sensitive. Determined by the properties of the element - the base.
- https://www.youtube.com/watch?v=qgzo40bfL1o
- All alloy materials exhibit characteristic metallic properties to one degree or another:
- shine;
- plastic;
- thermal conductivity;
- electrical conductivity.
- In addition, properties are divided into:
- Chemical, determined by the relationship of the material with chemically active substances.
- Mechanical, determined by interaction with other physical bodies.
- The main characteristics of alloy materials that influence their suitability for use in a particular engineering structure are:
- Strength is a characteristic of the strength to withstand mechanical loads and destruction.
- Hardness is the ability to resist the penetration of solid bodies into a material.
- Elasticity is the ability to restore the original shape of a body after deformation caused by external load.
- Plasticity is the opposite property of elasticity. Determines the ability of a material to change the shape of a body without its destruction under an applied load and maintaining this new shape.
- Viscosity - the ability to resist rapidly increasing (shock) loads
Hygroscopicity
How is this physical property of materials determined in materials science? Hygroscopicity is the ability to absorb water vapor and retain it inside itself as a result of capillary condensation. Directly depends on the relative humidity and air temperature, the size, type and number of pores of the substance, its nature.
If a material actively attracts water molecules with its surface, then it is called hydrophilic. If the material, on the contrary, repels them from itself, then it is called hydrophobic. In addition, some hydrophilic materials are highly soluble in water, while hydrophobic materials are resistant to aqueous media.
Technological properties of steel
Steel is considered one of the most common metals; its technological properties depend on its chemical composition; various impurities included in it can improve or worsen these characteristics.
- An increase in the carbon composition of steel significantly increases its hardenability, at the same time it reduces its suitability for forging. To perform this operation, as well as rolling, the carbon content should not exceed 1.4%.
- Adding manganese to steel significantly reduces the thermal conductivity of the material, which reduces its weldability. At the same time, with proper uniform heating (not too fast), such steels lend themselves well to forging.
- The use of nickel can improve the ductility of the alloy, so it facilitates forging. But one should take into account the fact that the same nickel forms stable scale during the heating process. It is not destroyed during forging, so it can be forged into metal, which will significantly reduce the quality of the product.
- An increase in chromium content leads to an increase in strength, so forging and rollingability of such alloys is satisfactory; there is a high probability of crack formation.
- Excess molybdenum leads to a decrease in thermal conductivity, which makes the steel very sensitive to the processing temperature; it should be heated and cooled in strict compliance with the technology. To forge these metals, it is necessary to use more powerful equipment.
- But the use of vanadium, on the contrary, improves malleability and makes the steel more resistant to overheating.
Negative impurities that significantly affect technological characteristics include sulfur and phosphorus. Excess of these substances can lead to red brittleness and cold brittleness, respectively. That is, steel with excess sulfur becomes brittle when heated, and if it contains a large amount of phosphorus, it will break at subzero temperatures. That is why, during steel smelting, many efforts are aimed at reducing these impurities in the metal, but, unfortunately, it is not possible to completely get rid of them.
As you can see, the chemical components of steel have a huge impact on its technological properties, therefore, when choosing a processing method, a thorough analysis of the composition of the alloy must be performed, otherwise problems may arise both in production and during operation of the product.
Basic definitions
It is necessary to clearly understand that metal alloys in most cases are formed without any human involvement at all. The fact is that it is possible to obtain a material that is absolutely pure from a chemical point of view only in the laboratory. Any metal that is used in everyday life probably contains traces of another element. A classic example is gold jewelry. Each of them contains a certain proportion of copper. However, in the classical sense, this definition still means a compound of two or more metals, which was purposefully obtained by man.
The entire history of man is an excellent example of how metal alloys were able to have a huge impact on the development of our entire civilization. It is no coincidence that there is even a long historical period called the “Bronze Age”.
Air resistance
Air resistance is the ability of a material to withstand repeated systematic drying and moistening for a long time without loss of its mechanical density, as well as without significant deformation.
Some materials begin to swell when periodically moistened, some shrink, and some warp too much. Wood, for example, is subject to alternating deformations. When cement is frequently moistened and dried, it tends to deteriorate and crumble.
Aluminum alloys
If the first half of the 20th century was the century of steel, then the second was rightly called the century of aluminum.
Aluminum alloys are divided into:
- Foundry (with silicon). Used to produce conventional castings.
- For injection molding (with manganese).
- Increased strength, with the ability to self-harden (with copper).
https://youtube.com/watch?v=5v8kGT8HK5c
Main advantages of aluminum compounds:
- Availability.
- Low specific gravity.
- Durability.
- Cold resistance.
- Good machinability.
- Electrical conductivity.
The main disadvantage of alloy materials is low heat resistance. When reaching 175°C, a sharp deterioration in mechanical properties occurs.
Another area of application is the production of weapons. Aluminum-based substances do not spark under strong friction and collisions. They are used to produce lightweight armor for wheeled and flying military equipment.
Aluminum alloy materials are widely used in electrical engineering and electronics. High conductivity and very low magnetizability make them ideal for the production of housings for various radio and communications devices, computers and smartphones.
Aluminum alloy ingots
The presence of even a small proportion of iron significantly increases the strength of the material, but also reduces its corrosion resistance and ductility. A compromise on iron content is found depending on the requirements for the material. The negative effect of iron is compensated by adding metals such as cobalt, manganese or chromium to the alloy composition.
Magnesium-based materials compete with aluminum alloys, but due to their higher price they are used only in the most critical products.
Water permeability
This physical property is the ability of materials to pass liquid through themselves under pressure. It is characterized by the volume of water that passes through 1 square meter in 1 hour. m of material under a pressure of 1 MPa.
It is important to note that completely waterproof materials are also available. These are steel, bitumen, glass, and the main types of plastics.
Methods for studying the structure of metals
The study of the structure of metals and alloys is carried out using macro- and microanalysis methods, the X-ray method, as well as flaw detection methods (X-ray, magnetic, ultrasonic).
The macroanalysis method is used to study the macrostructure, i.e. structure visible to the naked eye or with a magnifying glass. This reveals large defects: cracks, shrinkage cavities, gas bubbles, etc., as well as uneven distribution of impurities in the metal. The macrostructure is determined by the fractures of the metal, by macrosections (this is a sample of a metal or alloy, one of the sides of which is polished, thoroughly degreased, etched and examined with a magnifying glass with a magnification of 5–10 times).
Microanalysis reveals the structure of a metal or alloy using microsections, additionally polished to a mirror finish. The thin sections are examined in reflected light under an optical microscope at a magnification of up to 3000 times. Due to the different orientation of the metal grains, they are not etched to the same extent, and under a microscope, light is also reflected differently. Due to impurities, grain boundaries are etched more strongly than the base metal and are revealed more prominently. Knowing the microstructure, it is possible to explain the reasons for changes in the properties of the metal.
Using X-ray analysis, the atomic structure of metals, the types and parameters of crystal lattices, as well as defects lying in depth are studied. This analysis, based on the diffraction (reflection) of X-rays by rows of atoms in a crystal lattice, allows you to detect defects without destroying the metal. Instead of defects, X-rays are absorbed less than in solid metal, and therefore on photographic film such rays form dark spots corresponding to the shape of the defect.
The magnetic method is used to examine defects in magnetic metals (steel, nickel, etc.) at a depth of up to 2 mm. To do this, the product being tested is magnetized, its surface is coated with iron powder, the surface is inspected, and the product is demagnetized. A non-uniform field is formed around the defect, and the magnetic powder follows the contours of the defect. The ultrasonic method provides effective control of the quality of metal products and workpieces of almost any size. In pulsed ultrasonic flaw detectors, the ultrasonic wave from the probe-emitter propagates in the test product and, when it encounters a defect, is reflected from it. In this case, the reflected waves are received, amplified and transmitted to the indicating indicator.
Metal classification
In nature, there are several types of metals that differ in their properties, characteristics and appearance. Each variety behaves differently when interacting with other materials or under the influence of environmental factors.
Types of metals
Black
This group includes iron and alloys based on it. Characteristic features of ferrous metals:
- high density;
- the melting point is much higher than that of representatives of other groups;
- color - dark gray.
Representatives of the group of ferrous metals include: tungsten, chromium, cobalt, molybdenum, iron, nickel, titanium, manganese, uranium, neptunium, plutonium and others. They are used in various industries and have different properties. Steel and cast iron are considered popular.
The composition of ferrous metals includes not only iron, but also various impurities, which include sulfur, phosphorus or silicon. They contain different amounts of carbon in their composition.
Colored
Representatives of this group are more in demand. This is due to the fact that non-ferrous metals are used in more industries. They can be used in mechanical engineering, advanced technologies, radio electronics, and metallurgy. Key features of non-ferrous metals:
- low melting point;
- large color spectrum;
- good ductility.
Due to the low strength of representatives of the color group, they are used in conjunction with different types of denser materials. Representatives of this group: magnesium, aluminum, nickel, lead, tin, zinc, silver, platinum, rhodium, gold and others.
Soft
It is possible to distinguish certain types of metals, which will belong to the group of hard and soft. The soft ones are:
- Aluminum - has corrosion resistance, light weight, good ductility. It is used in the electrical industry, in the construction of aircraft and in the manufacture of dishes.
- Magnesium is a lightweight material that is susceptible to corrosive processes. To get rid of this drawback, it is used in alloys with other materials.
These are key representatives of the group of soft metals.
Solid
Popular materials in this group are:
- Tungsten is considered the most refractory metal. In addition to this, it is one of the most durable. Resistant to chemical influences.
- Titanium - the fewer inclusions of other materials in this metal, the stronger it becomes. It is used in the construction of cars, rockets, airplanes, ships, as well as in the chemical industry. It is well processed under pressure and is not susceptible to corrosive processes.
- Uranium is another metal considered one of the strongest in the world. It is radioactive and is used in various industries.
Representatives of the “hard group” are less amenable to processing and are used in fewer areas of human activity than soft ones.
Frost resistance
An important physical property in Russian realities. This is the ability of a material saturated with water to withstand repeated alternating freezing and thawing without a significant decrease in strength or the appearance of visible signs of destruction.
Destruction in this process is often due to the fact that when water freezes, it increases in volume by about 9%. Moreover, its greatest expansion upon transition to ice is observed at -4 °C. When the pores of a material are filled with water, it expands and freezes, the pore walls experience significant damage, which leads to the destruction of the material.
Accordingly, frost resistance will determine the degree of saturation of the pores with water and its density. It is dense materials that are considered frost-resistant. Of the porous ones, only those that are characterized by a large presence of closed pores can be included in this category. Or whose pores are filled with water by no more than 90%.
Physical properties can represent the important capabilities of materials. We have already discussed some of them in detail in the article. This is the ability to withstand cold, repeated filling with water and drying, retain, absorb, release liquid and other important characteristics.
What species are found?
The properties of metals largely depend on what type a particular ingredient belongs to. From this perspective, it is worth highlighting the black and colored components.
Chermet
This group is considered the most common and in demand from a volumetric perspective. They got their name due to their dark color. At the same time, a distinctive feature of ferrous ores is their low cost.
In turn, it is classified into:
- iron - this includes iron-containing materials and bases, as well as nickel and cobalt alloys;
- refractory bases for alloys (have a melting point equal to or exceeding 1600 degrees Celsius, which is a fairly high indicator);
- low-strength rare earth elements, such as cerium, neodymium and others (actively used in the production of microelectronics).
Tsvetmet
It is generally accepted that this group of elements is characterized by lower strength characteristics, melting point, resistance to mechanical loads, but a more respectable cost. It is clear that there are exceptions to all these positions.
People of color are ranked into the following categories:
- Lungs - lithium, sodium and so on. They are characterized by low density - up to 5 tons per cubic meter. This is only 5 times more water.
- Heavy - lead, silver, gold. Their density is several times higher than that of lungs.
- Noble ones are the same gold and silver, as well as platinum and plutonium.
“Colored” varieties can also be divided into refractory and low-melting.
Moisture release
This is the ability of a material to release moisture into the environment. When exposed to air, raw materials and products retain their moisture only under certain conditions - at relative equilibrium air humidity. If the indicator is below this value, then the material begins to release moisture into the atmosphere and dry out.
The speed of this process depends on several factors: on the difference between the humidity of the material itself and air humidity (the higher it is, the more intense the drying), on the properties of the material itself - its porosity, nature, hydrophobicity. Thus, a raw material with large pores, hydrophobic, will release liquid more easily than a hydrophilic material with small pores.
Structure of a mechanical ingot
The shape of the growing crystals is determined by:
- the conditions of their contact with each other;
- alloy composition;
- presence of impurities;
- cooling mode.
The mechanism of crystal formation is dendritic (tree-like) in nature. Dendritic crystallization is characterized by the fact that the growth of nuclei occurs at an uneven rate. After the formation of nuclei, their development proceeds in those planes and lattice directions that have the highest packing density of atoms and the minimum distance between them. In these directions, long branches of the future crystal—first-order axes—are formed. From the axes of the first order, new axes of the second order begin to grow, from the axes of the second order, axes of the third order, etc.
Steel ingots are produced by cooling in metal molds (molds) or in continuous casting plants. In a mold, steel cannot harden simultaneously throughout its entire volume, since it is impossible to create a uniform rate of heat removal. Therefore, the process of steel crystallization begins at the cold walls and bottom of the mold and spreads into the liquid metal. When the liquid metal comes into contact with the walls of the mold, a zone of small equiaxed crystals is formed at the initial moment. Since the volume of solid metal is less than liquid, an air gap forms between the wall of the mold and the solidified metal and the wall itself heats up from contact with the metal, so the cooling rate of the metal decreases and the crystals grow in the direction of heat removal. In this case, a zone is formed consisting of tree-like (columnar) crystals.
In the inner zone of the ingot, as a result of slow cooling, equiaxed, non-oriented crystals of large sizes are formed. In the upper part of the ingot, which solidifies last, a shrinkage cavity is formed, since upon cooling the volume of the metal decreases. Under the shrinkage cavity, the metal turns out to be loose due to the large number of shrinkage pores.
To obtain products, only part of the ingot is used, removing the shrinkage cavity and loose metal of the ingot for subsequent remelting.
Water absorption
If we talk briefly about the physical properties of building materials, we cannot fail to mention water absorption - the ability to retain and absorb liquid. The property is characterized by the volume of water absorbed by a dry material when it is completely immersed in water. Expressed as a percentage of the mass (material).
Water absorption will be less than the true porosity of the product, since a certain number of pores in it remain closed. Therefore, it will vary depending on their number, volume, and degree of openness. The value will also be influenced by the nature of the material and its hydrophilicity.
As a result of saturation of the material with water, its other physical properties sometimes change significantly: thermal conductivity and density increase, volume increases (typical of clay, wood), strength decreases due to the disruption of bonds between individual particles.
Main types of alloys
The most numerous types of metal alloys are made based on iron. These are steels, cast irons and ferrites.
Steel is an iron-based substance containing no more than 2.4% carbon, used for the manufacture of parts and housings for industrial installations and household appliances, water, land and air transport, tools and devices. Steels have a wide range of properties. The common ones are strength and elasticity. The individual characteristics of individual steel grades are determined by the composition of alloying additives introduced during smelting. Half of the periodic table is used as additives, both metals and non-metals. The most common of them are chromium, vanadium, nickel, boron, manganese, phosphorus.
Alloy steel
If the carbon content is more than 2.4%, such a substance is called cast iron. Cast iron is more brittle than steel. They are used where it is necessary to withstand large static loads with small dynamic ones. Cast iron is used in the production of frames for large machine tools and technological equipment, bases for work tables, and in the casting of fences, gratings, and decorative items. In the 19th and early 20th centuries, cast iron was widely used in building structures. Cast iron bridges have survived to this day in England.
Cast iron radiators
Substances with a high carbon content and having pronounced magnetic properties are called ferrites. They are used in the production of transformers and inductors.
Copper-based metal alloys containing from 5 to 45% zinc are commonly called brasses. Brass is slightly susceptible to corrosion and is widely used as a structural material in mechanical engineering.
Yellow brass
If you add tin to copper instead of zinc, you get bronze. This is perhaps the first alloy deliberately obtained by our ancestors several thousand years ago. Bronze is much stronger than both tin and copper and is second in strength only to well-forged steel.
Lead-based substances are widely used for soldering wires and pipes, as well as in electrochemical products, primarily batteries and accumulators.
Two-component aluminum-based materials, which contain silicon, magnesium or copper, are characterized by low specific gravity and high machinability. They are used in the engine, aerospace, and electrical component and appliance industries.
Physical properties of metals
Among the main general physical properties of metals are:
- Melting.
- Density.
- Thermal conductivity.
- Thermal expansion.
- Electrical conductivity.
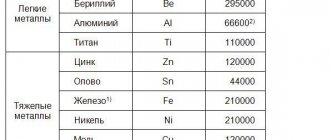
An important physical parameter of a metal is its density or specific gravity. What it is? The density of a metal is the amount of substance contained in a unit volume of the material. The lower the density, the lighter the metal. Light metals are: aluminum, magnesium, titanium, tin. Heavy metals include such metals as chromium, manganese, iron, cobalt, tin, tungsten, etc. (in total there are more than 40 types).
The ability of a metal to change from a solid to a liquid state is called melting. Different metals have different melting points.
The rate at which heat is conducted in a metal when heated is called the thermal conductivity of the metal. And compared to other materials, all metals have high thermal conductivity; to put it simply, they heat up quickly.
In addition to thermal conductivity, all metals conduct electric current, although some do it better and some worse (this depends on the structure of the crystal lattice of a particular metal). The ability of a metal to conduct electric current is called electrical conductivity. Metals with excellent electrical conductivity are gold, aluminum and iron, which is why they are often used in the electrical industry and instrument making.
Water permeability
Water permeability is the ability of a material to give off liquid when dried and absorb water when wet.
When studying the physical properties of materials, you need to pay attention to the fact that saturation with water can occur in two ways: when exposed to a substance in a liquid state or when exposed to only its vapor.
This leads to two other important properties: hygroscopicity and water absorption.
Alloy theory
A metal alloy is a material obtained by fusing two or more metals or metals with non-metals and having metallic properties. The substances that form an alloy are called components.
A phase is a homogeneous part of an alloy, characterized by a certain composition and structure and separated from other parts of the alloy by an interface. Structure refers to the shape, size and nature of the relative arrangement of phases in metals and alloys. Structural components are separate parts of an alloy that have the same structure with their characteristic features.
Types of alloys by structure. According to the nature of the interaction of the components, all alloys are divided into three main types: mechanical mixtures, chemical compounds and solid solutions.
A mechanical mixture of two components A and B is formed if they are not capable of interaction or mutual dissolution. Each component crystallizes into its own crystal lattice. The structure of mechanical mixtures is heterogeneous, consisting of separate grains of component A and component B. The properties of mechanical mixtures depend on the quantitative ratio of the components: the more of a given component in the alloy, the closer the properties of the mixture are to its properties.
A chemical compound is formed when alloy components A and B react chemically. Moreover, the ratio of the numbers of atoms in the compound corresponds to its chemical formula AmBn. A chemical compound has its own crystal lattice, which differs from the crystal lattice of its components. Chemical compounds have a homogeneous structure, consisting of grains of identical composition and properties.
When a solid solution is formed, atoms of one component enter the crystal lattice of another. Substitutional solid solutions are formed as a result of partial replacement of atoms of the crystal lattice of one component with atoms of the second (Fig. 6, b).
Interstitial solid solutions are formed when atoms of a dissolved component are introduced into the crystal lattice of a solvent component (Fig. 6, c). The solid solution has a homogeneous structure, one crystal lattice. Unlike a chemical compound, a solid solution does not exist at a strictly defined ratio of components, but in a concentration range. Solid solutions are designated by lowercase letters of the Greek alphabet: α, β, γ, δ, etc.