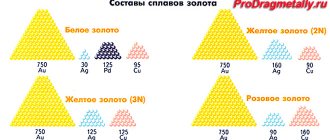
Of the non-ferrous metals in their pure form, only aluminum and copper are used in construction work and industry. They have excellent characteristics suitable for the typical type of work. However, alloys based on these materials are becoming increasingly popular. One of the alloys of copper is brass. Brass is a multi-component or consisting of only two components copper-based alloy, in which the main element is the alloying component - zinc and additive components such as nickel, tin, manganese, lead, iron and others are rarely used.
Brass weight table
Specific gravity of coke and its weight depending on units of measurement
| Material | Specific gravity (g/cm3) | Cube weight (kg) |
| Casting type brass | From 8.3 to 8.5 | From 8300 to 8500 |
| Casting type brass in ingots | From 8.3 to 8.5 | From 8300 to 8500 |
| Pressure treated brass | From 8.2 to 8.85 | From 8200 to 8850 |
Application of brass alloy
A relatively inexpensive and easy way to obtain the alloy, its unique properties have allowed it to become universal, so it has many areas of application. Rods and wire are drawn from it, stamped into sheets, and also made into very thin foil. Small and large parts, fittings, pipes, fittings are used in many industries:
- Automotive and chemical industry
- Instrumentation
- Jewelry
- In aircraft construction, creation of sea and river vessels
The color of the alloy is very similar to gold, so in jewelry it is often used to make jewelry and is highly polished. When a real master gets down to business, it is difficult for an ordinary person to understand that this is a base metal. Brass jewelry looks beautiful and expensive.
In its pure form, copper is very unstable to corrosion , and zinc is a brittle metal; with the help of an alloy of these two types of metal, their best properties were combined and disadvantages were minimized.
- The deformed variety is tombac, which is characterized by high strength, low friction force and resistance to rust.
- Foundry - shaped products are made from it by casting, as well as semi-finished products. It contains 50-81% copper. This type does not rust, has high mechanical properties, is easy to handle due to its liquid state, and is resistant to friction with other materials.
- Automatic brass - due to its softness, it is used to make rods, sheets, tapes and strips.
- Jewelry alloys.
Advantages of brass
Brass has proven itself to be an elastic alloy with high corrosion resistance. Parts made from this material are durable and reliable in use.
Brass is especially valued in the refrigeration and food industries, due to the smooth and efficient operation of equipment made with this material, as well as a significant reduction in costs compared to the use of copper. Brass is also often used in the automotive, shipbuilding and aircraft industries.
If we talk about construction work, then brass is widely used in the production of sanitary products, in engraving work, as well as for finishing facades and furnishing the interiors of buildings.
Among the main advantages, it is also worth highlighting the following:
Brass: density and properties
Brass has been known to people since ancient times; in appearance, the alloy resembles gold , but is much cheaper.
Due to its properties, it immediately found wide application; the alloy was discovered for the first time in Ancient Rome, and then again in the 18th century. Its appearance resembles a noble metal, but there is no gold in it; the basis is an alloy of zinc and copper and some other elements, the share of which is no more than 10%. Since copper contains a lot of zinc and copper, its characteristics are very similar to these elements. In color, the alloy can change from light yellow to red shades. Density is 8300-8700 kg/m3 . The melting point of brass is 880-950°C, it depends on its composition; if it contains more zinc, then the melting point decreases. By its density, brass belongs to the group of non-ferrous metals and alloys.
With resistance welding, brass can be easily welded and rolled well. If its surface is not varnished, it quickly turns black in air, but when combined with other metals, it has greater air resistance than, for example, copper, and is very easily polished.
Brass alloy is easy to work in cold and hot conditions and has good mechanical characteristics. In appearance it is very similar to copper , but unlike copper, brass has high wear resistance and strength. Brass is less refractory, but easier to process because it is more malleable and tough.
The heat and electrical conductivity of brass will depend on the content of the base metal; when the proportion is higher, then these properties are more pronounced.
Mechanical and physical properties
Brass LS 59 (or LS59-1) is characterized by relatively high hardness - HB 10-1=150-160 MPa, while the melting point of the alloy is at 900°C. The remaining properties of this material are presented in the table:
Since this brass alloy is initially intended for pressure processing, the type and characteristics of the latter can determine the subsequent performance properties of the brass product. Thus, the characteristics of hot deformation, namely the magnitude of pressure, can make the alloy hard, semi-hard or soft. The use of hot annealing additionally gives the alloy greater corrosion resistance and wear resistance.
Despite the fact that we are dealing with a multicomponent substance processed by pressure, the main practical name for LS59 brass is an automatic alloy. This is due to the inherent good electrical and thermal conductivity of the material. As for corrosion resistance, after proper processing, thanks to lead, the LS59-1 alloy becomes more resistant to cracking that occurs due to increased humidity or ambient temperature. In this respect, this grade is significantly superior to alloys L68 and L63.
Basic information about copper
Deliver alloy Lazh60-1-1 (GOST 15527 - 2004) in St. Petersburg
Copper is the most common non-ferrous metal. It received its name in Latin - Cuprum - in honor of the island of Cyprus. It was mined there by the ancient Greeks thousands of years ago. Historians even came up with the Copper Age, which lasted from the 4th to 5th centuries BC. e. At that time, people made from popular metal:
- weapon;
- dishes;
- decorations;
- coins.
In the table D.I. Mendeleev, it ranks 29th. This element has unique properties - physical, chemical and mechanical. In ancient times, copper could be found in the natural environment in the form of nuggets, sometimes of very large sizes. People heated the rock over an open fire and then cooled it sharply. As a result, it cracked, which made it possible to restore the metal. This simple technology made it possible to begin the development of a popular element.
Improved performance
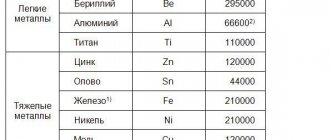
To improve such properties of brass alloys as density, color, hardness, anti-corrosion resistance and others, in addition to copper and zinc, alloying elements are added, which include tin, iron, arsenic, aluminum, nickel, manganese, etc. Quantity There are very few such elements added to the composition of brass. As a rule, it does not exceed a few percent. The most significant properties that can be improved by alloying brass are cavitation density, wear resistance and corrosion resistance.
The diagrams show how much stronger and harder brass is than copper due to alloying elements
Alloying elements added to the chemical composition of brass have different effects on its properties. Thus, silicon, when its content in the alloy is exceeded, reduces its density and, accordingly, worsens its strength characteristics. If, in addition to silicon, lead is added to brass, it will be painted a beautiful color, and its anti-friction properties will increase.
To improve the tensile strength properties of brass, tin, aluminum or manganese are added to its composition. If brass is alloyed with manganese and iron, the amount of which should not exceed 2–3%, its elongation coefficient can be significantly improved. Typically, other chemical elements used to alloy brass worsen this indicator.
Physical properties of simple brasses (click to enlarge)
To increase the corrosion resistance of brass, elements such as nickel, aluminum, tin and manganese are added to its composition. Particularly noteworthy is nickel-plated brass, which is called white because of its color. Due to the nickel content in their composition, the surface of products made from such alloys is not subject to cracking even when used in conditions of high humidity.
Adding tin to the composition of brass allows you to increase its density and, accordingly, its property such as strength. Products made from such alloys can be successfully used in salt water. Among the wide variety of brass brands, there are those specially created for use in conditions of constant exposure to sea water.
Chemical composition and examples of the use of brasses containing tin (click to enlarge)
Lead is added to brass mainly to ensure good machinability. This element ensures the formation of short and easily broken chips during processing on turning, milling or drilling equipment. In addition, the lead content when processing brass with metal-cutting tools guarantees a surface with low roughness values.
A rather rare element with which brass is alloyed is arsenic. Products made from such brass are successfully used in fresh, highly aggressive liquid environments at normal or elevated temperatures. If iron and nickel are added to the chemical composition of brass alloyed with arsenic, then products made from it can be successfully used in acidic and alkaline environments.
Suitable for cutting, including laser cutting, of brass with a zinc content of less than 42%
Marking order
The following markings are accepted. The brass alloy is designated by the letter “L”, followed by the letters of the main elements that form the alloy. In grades of wrought brass, the first two digits after the letter “L” indicate the average copper content as a percentage. For example, L70 is brass containing 70% Cu. In the case of alloyed deformable brasses, letters and numbers are also indicated indicating the name and amount of the alloying element, LAZH60-1-1 means brass with 60% Cu, alloyed with aluminum (A) in the amount of 1% and iron (I) in the amount of 1%. The Zn content is determined by the difference from 100%. In cast brasses, the average percentage of alloy components is placed immediately after the letter indicating its name. For example, brass LTs40Mts1.5 contains 40% zinc (Z) and 1.5% manganese (Mts). Used in pipes
Marking order
To mark the alloy in question, certain rules were adopted to indicate the concentration of the main substances. All brands of brass begin with the designation “L”, which may be followed by the letters of the chemical substances included in the composition.
A wrought brass alloy or another variety of it has a number after the first letter characterizing the percentage of copper. In addition, the marking may indicate the concentration of alloying elements, for which the “L” sign comes with other letter designations.
To indicate the concentration of alloying elements, a dash is placed after the main number, then the percentage of the following elements is indicated. A dash is also used to separate digital symbols. The concentration of the second main element (zinc) is calculated by subtracting other indicators of the concentration of copper and alloying elements from 100% of the value. An example of how brass is designated according to established standards is the marking LAZH70-1-2. It should be read as follows:
- The alloy contains 70% copper.
- The alloying elements are aluminum and iron, the concentration of which is 1% and 2%, respectively.
- Zinc concentration: 100 – 70 – 1 – 2 = 27%.
In some cases, the concentration of zinc is indicated by the corresponding letter, and the amount of copper is calculated. A similar marking method is more often used to mark foundry brass.