Zinc or Zincum is element 30 of Mendeleev's periodic table of chemical elements and is designated by the symbol Zn. It is mainly used in the creation of deformed semi-finished products and as part of various types of mixtures. In its pure form, it looks like a brittle metal of a bluish-silver color; it quickly oxidizes and becomes covered with a protective film (oxide), due to which it noticeably tarnishes.
It is mined in Kazakhstan, Australia, Iran and Bolivia. Due to the difficulties in identifying the metal, it is often called "blende" .
Historical reference
The name “zinc” itself was first mentioned in the book “Liber Mineralium” by Paracelsus.
According to some sources, it meant “prong.” The alloy of zinc with copper or brass has been known for a long time. It was used in Ancient Greece, India and Ancient Egypt, and later the material became known in China. The metal was obtained in its pure form only in the first half of the 18th century in 1738 in Great Britain using the distillation method. Its discoverer was William Champion. Industrial production began 5 years later, and in 1746 in Germany, the chemist Andreas Sigismund Marggraff developed and described in detail his own method for producing zinc . He proposed using the method of calcining a mixture of metal oxide and coal in fireproof clay retorts without air access. Subsequent condensation of the vapors had to take place in the refrigerator. Due to his detailed description and painstaking development, Marggraf is often called the discoverer of the substance.
At the beginning of the 19th century, a method was found for isolating metal by rolling at 100 C o -150 C o. At the beginning of the next century, they learned to extract zinc using the electrolytic method. In Russia, the first metal was produced only in 1905.
Physical properties
- Atomic number: 30.
- Atomic mass: 65.37.
- Atomic volume: 9.15
- Density: 7.133 g/cm3.
- Temperature required for melting: 419.5 C o.
- Boiling point: 906 C o.
- Surface energy: 105 mJ/m2.
- Specific electrical conductivity: 16.2*10-6 S/m.
- Molar heat capacity: 25.4 J/(K*mol).
- Molar volume: 9.2 cm3/mol.
Zinc has weak mechanical properties; at normal temperatures it easily breaks and crumbles, but at temperatures of 100 C o -150 C o it becomes quite malleable and is easily deformed: it is forged and rolled into sheets. Plain water is safe for metal, but acids and alkalis easily corrode. Because of this, zinc in its pure form is not used for the manufacture of parts, only alloys.
Properties of zinc
Zinc is in the second group of the periodic table. Like other elements in this group, it is divalent and has pronounced metallic properties. But as a metal, zinc is inferior in activity to beryllium, magnesium and alkaline earth metals, which represent the main subgroup of the same second group.
The serial number of zinc is 30, in the fourth row it is on the boundary between nickel and copper on the one hand and gallium, germanium and arsenic on the other. This transitional position between typical metals and non-metals explains the appearance of non-metallic properties in zinc, expressed in the amphotericity of its oxide.
The melting and boiling points of zinc are 427 and 907 ºС, respectively. The relatively low boiling point was the reason that delayed the development of zinc production. Conventional methods of smelting metals by heating a mixture of ore and coal did not produce results due to the volatility of zinc, which left the furnace space with flue gases in the form of vapors. Later they learned to condense vapors, which gave rise to the distillation method for producing zinc.
Natural zinc with an atomic mass of 65.37 consists of five isotopes: Zn64, Zn66, Zn67, Zn68, Zn70.
Electrochemical potential of zinc
Zn2+ + 2e = Zn; E0 = -0.76 V.
A large negative potential value characterizes high activity of zinc. However, it decomposes cold water; The reason for this is not only the thin film of basic carbon dioxide salts covering the metal, but also the slow discharge of hydrogen ions on zinc - a high hydrogen overvoltage on it.
Impurities of iron, copper and other more electropositive metals significantly accelerate the dissolution of zinc in acids.
To protect iron from corrosion, it is coated with a layer of zinc. With local destruction of the coating, the protection continues: exposed areas of iron are not destroyed, they become sites for the release of hydrogen due to the dissolution of zinc.
In strong acids, zinc dissolves with the release of hydrogen, and in alkali solutions - with the formation of zinc acid anions, zincates:
Zn + 2NaOH = Na2ZnO2 + H2.
Zinc oxide ZnO is a loose white powder obtained by burning zinc vapor in air; it is widely used for the production of oil and other paints: its pure white color and hiding power have made zinc white a necessary material in the painting industry.
Zinc oxide is an infusible substance: at temperatures above 1800 ºС it evaporates without melting. The temperature at which the reduction of zinc from carbon monoxide begins is about 950 ºС.
Zinc sulfide ZnS is also infusible and noticeably volatile at temperatures above 1180 ºC. When heated in air, ZnS is oxidized to basic sulfates - ZnO · nZnSO4, ZnSO4 sulfate and ZnO oxide.
Chemical properties
The external electronic configuration of one zinc atom can be written as 3 d 104 s 2. The metal is active and is an energetic reducing agent.
At a temperature of 100 C, it becomes covered in open air with a film consisting of basic carbonates and becomes very dull. When exposed to carbon dioxide and high humidity, the element begins to deteriorate. In an oxygen or normal environment, when exposed to high heat, zinc burns, producing a bluish flame and white smoke, which consists of zinc oxide. The dry elements of fluorine, bromine and chlorine have a flammable effect on zinc, but only with the participation of water vapor. When a metal and strong mineral acids combine, the former dissolves, especially if the mixture is heated, resulting in the formation of the corresponding salts . Alkalis, melts and solutions oxidize the substance, resulting in the formation of zincites, soluble in water, and hydrogen is released. The intensity of the effects of acids and alkalis depends on the presence of impurities in zinc. The more “clean” the metal, the weaker it interacts due to hydrogen overvoltage.
Casting process
Bronze casting begins with preparing the workplace. Install a stand under the flask and a crucible. It is made of fire-resistant material. You can pour a layer of sand into a tray or use a metal plate.
Before loading the crushed scrap into the furnace, it is necessary to prepare the flask. It is heated well and kept for at least 2 hours at +600°C. The refractory crucible at this temperature begins to emit a yellowish glow. While the heated mold is cooling, begin melting the crushed scrap.
The melting pot is filled to 1/3 of its volume. Place the pieces of scrap in a hot oven and leave until completely melted. The crucible or other melting vessel is set to heat when the temperature in the furnace is close to or has reached the melting point of bronze. Tin bronzes become liquid up to 1000°C; tin-free bronzes have to be heated longer and have a higher melting point.
When all the bronze has melted, it is left in the oven for 3-5 minutes so that the melt warms up well and becomes less viscous. Then the melting pot is removed with tongs or a special hook. The melt is ready. It's time to start making the casting.
Pouring bronze into a mold
The molten metal is poured into the hole of the mold in a thin stream; the bronze should evenly fill all the voids. It compacts under its own weight. To ensure that the hot form is filled well, it is placed on a rotating stand with a manual or electric drive. This trick is necessary to obtain high-quality casting. If you pour bronze calmly, the corners of the casting will be rounded. Spin the mold with the hot melt during filling. Once the bronze sets, the casting shape cannot be changed. In factory conditions, technological centrifuges are used. At home, craftsmen make similar industrial installations based on used washing machines.
It is necessary to impart movement to the alloy when making small complex castings. The bronze melt will not have enough of its own weight to fill all the voids. It will harden unevenly, and cavities and folds will appear on the surface of the casting.
How to make a casting mold
Independent production of a casting mold begins with the selection of a body into which the future casting will fit freely and half of the space will remain free. This can be a tin can or a specially made container. Traditionally, the size of the flask is 1.3–1.5 times greater than the dimensions of the casting. This ratio is necessary so that the sand-clay mixture forms an even layer on all sides of the part. The filling for the flask is made from materials that can retain heat. The cast workpiece will only be of high quality if it cools gradually.
The form is made of two parts:
- top frame (occupies at least 1/4 of the height of the form);
- the bottom drawer, the future part can easily fit into it.
For packing, make a mixture of sand and clay in a 3:1 ratio. To accumulate heat, 1/5 of the coal chips is added to the clay; it is better to use hard coal, it has a higher heat capacity. A homogeneous mass is placed in both parts of the flask. The mixture should not be compacted too much so that the clay does not cake and remains loose while heating in the oven. Before placing the part in the prepared bottom box, it is thickly coated with talcum powder or graphite powder. Then a hole is made to pour bronze, heated to the melting point.
The finished form must be dried before use.
The product is removed from the mold only after cooling. The mold is carefully disassembled, then the casting is removed.
Technology for obtaining higher quality castings
It is difficult to obtain castings of the required geometry using a homemade method. You need to be prepared for the fact that the casting will have to be polished, ground, and brought to the required dimensions for a long time. The process of melting bronze at home is associated with a number of limitations. Nuances that it is advisable to take into account when making bronze castings yourself:
- it is better to use a muffle furnace for melting, equipped with a thermostat; manufacturers offer small installations for making home castings, operating from a standard 220 V network;
- when making a flask, it is necessary to take into account the difficulty of filling it with a melt; in places of possible voids, allowances are made for future finishing of the part;
- Instead of sand-clay flasks for small parts, plaster molds are made with wax or paraffin filling. When infused, hot bronze is displaced not by air, but by molten stearin.
Paraffin molds are made on the basis of plaster casts made from the future part. In this case, the accuracy of casting production increases. Gypsum flasks are convenient for small castings; they can be made one-piece. True, the process of their manufacture is more complicated: first, a mold for a paraffin model is divided, and then it is filled with molding plaster. It is convenient to place such structures in a centrifuge.
When starting to melt bronze scrap at home, you should take into account the composition of the alloy. It depends on the chemical composition of bronze. The viscous molten bronze slowly fills the flask. To avoid voids, the filled form is spun in a centrifuge. Under the influence of centrifugal force, metal heated to the melting point is distributed evenly. The casting is of high quality.
Content in nature
Zinc does not occur as an independent element in nature. It can be extracted from 66 minerals, including sphalerite, calamine, franklinite, zincite, willemite, and smithsonite. The former is the most common source of the metal and is often called "zinc blende". It consists of zinc sulfide and impurities that give the mineral its varied colors. This makes it difficult to find and correctly identify.
You can find zinc in acidic and igneous rocks - in the latter there is a little more of it. Often the metal in the form of sulfide, together with lead is found in thermal waters and migrates in surface and underground sources.
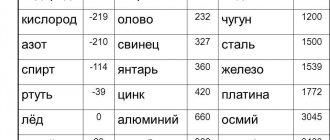
Melting point and specific heat capacity of zinc
The melting point of zinc is one of the lowest among metals used in industry – 420 °C. At lower temperatures, tin (232 °C) and lead (327 °C) melt.
The melting point of zinc is one of the lowest.
By adding tin to zinc, you can lower the melting point to 199 °C, and in combination with tin and lead - to 150 °C. The melting point of alloys is lower than that of each individual element.
A little history
R. Maggraf was the first to develop a method for obtaining pure zinc.
The metal was isolated in its pure form in laboratory conditions by distillation. This happened in Great Britain in 1738. After numerous experiments, in 1743 the British developed an industrial method for producing zinc. Three years later, in Germany, zinc was obtained from its salts by calcining them without access to oxygen. The resulting metal vapor condensed in the refrigerator. Later, electrochemical technology and technology for producing metal by rolling when heated to 100–150 °C were developed.
In Russia, the first zinc ingot was obtained in 1905. Ancient people, not knowing about the existence of zinc, noticed that the inclusion of this element along with lead in copper ore significantly reduces the melting point of copper. Therefore, at a certain historical stage, copper turned out to be more in demand than iron, which melts at a higher temperature.
Description and physical characteristics of zinc
- Zinc does not occur in nature in the form of nuggets. To date, 66 types of minerals containing this element are known. Most often they are found in acidic and volcanic rocks, in thermal waters, and can lie in deep layers of the earth’s crust, from where they are carried away by groundwater.
- The most common mineral is “zinc blende”. It contains zinc sulfide and various impurities. Impurities give minerals different color shades, so it is easy to be “deceived” and incorrectly recognize this mineral, which complicates its search and extraction.
- A fresh ingot has a silvery color, but very quickly, like aluminum, lead, and tin, it becomes covered with an oxide film and becomes dull.
- The specific heat of fusion of zinc is 122 kJ/kg. It determines the amount of energy required to melt 1 kg of zinc heated to its melting point. The specific heat of fusion of zinc is quite high, it is 2 times greater than that of tin and 5 times greater than that of lead.
- The specific heat capacity of zinc is 400 J/(kg*°C). The value determines the amount of energy to increase the temperature of a metal weighing 1 kg by 1 °C. It coincides with the specific heat capacity of copper and brass. For lead and tin, the specific heat capacity is equal to 140 and 230 J/(kg*°C), respectively.
The specific heat of fusion of zinc is 122 kJ/kg.
The given values show that melting zinc requires high energy consumption.
Application of zinc and its alloys
Zinc is one of the most sought after elements. It ranks 3rd in terms of production volume among non-ferrous metals. The championship belongs to copper and aluminum. But in its pure form it is rarely used: at high temperatures the metal becomes soft, and at low temperatures it becomes brittle. The process where it is used in its pure form is the recovery of precious metals from pure lead.
About 40% of the metal produced is used to protect steel structures from mechanical damage and corrosion. The high degree of anti-corrosion protection is explained by greater chemical activity than iron. Therefore, the zinc layer reacts earlier than the main structure.
Previously, printing houses used zinc ink for printing. Currently, it is classified as a harmful substance and other dyes are used instead. But zinc white is still used today, although its production is declining. Their main advantage is their high adhesion to the painted surface, providing high-quality coating. Whitewash is resistant to ultraviolet radiation and retains its color for a long time.
https://www.youtube.com/watch?v=o74G0zUgqTY
Zinc dust is used in pyrotechnics and fireworks. It gives the flame a bluish tint.
Zinc is widely used by humans.
Zinc alloys are much more often used. They have a high melting point and mechanical strength. Additives include copper, lead, tin, aluminum, and magnesium.
Alloys are widely used in the automotive and aviation industries, and in the production of household appliances.
Current sources with electrodes made of mercury-zinc and silver-zinc alloys have become widespread.
Features of smelting
The temperature required to melt zinc should be less than 419 C o, but not more than 480 C o.
Otherwise, metal waste will increase and wear on the walls of the bath, which is usually made from iron, will increase. In the molten state, no more than 0.05% iron admixture is allowed, otherwise the temperature required for melting will begin to rise. If the percentage of iron content exceeds 0.2%, zinc cannot be rolled. Zinc is obtained from polymetallic ores, which can contain up to 4% of the element . If the ores have been enriched by selective flotation, up to 60% of zinc concentrates can be obtained from them, the rest will be occupied by concentrates of other metals. Zinc concentrates are fired in furnaces in a fluidized bed, after which zinc sulfide turns into oxide and sulfur dioxide is released. The latter is consumed: sulfuric acid is obtained from it.
To convert zinc oxide into the metal itself, two methods are used.
- Distillation or pyrometallurgical. The concentrate is fired, then sintered to impart gas permeability and granularity and reduced with coke or coal at temperatures of 1200-1300 C°. During the reaction, metal vapors are formed, which are condensed and poured into molds. The purity of zinc reaches 98.7%, after which it can be increased to 99.995% using rectification, but the latter method is quite expensive and complex.
- Electrolytic or hydrometallurgical. The fired concentrates are treated with sulfuric acid, the solution is cleaned of impurities using zinc dust and subjected to electrolysis in bathtubs lined with lead or vinyl plastic. Zinc is deposited on aluminum cathodes, from where it is collected and melted in induction furnaces. The purity of the metal obtained by this method reaches 99.95%.
Raw materials for zinc production
The main sources of zinc are sulfide, copper-lead-zinc, copper-zinc and lead-zinc ores.
In sulfide ores, zinc is usually present in the form of sphalerite or wurtzite, the composition of which corresponds to the formula ZnS, and marmatite nZnS · mFeS. Companions of zinc in polymetallic ores are minerals and elements.
In the oxidized zones of zinc-containing ore deposits, the main oxygen-containing zinc minerals are: smithsonite ZnCO3, zincite ZnO and calamine ZnO·SiO2·H2O. Oxidized zinc ores are currently of secondary importance.
In sulfide polymetallic ores, the zinc content is usually 1...3%. These ores have a complex composition. All this necessitates the preliminary enrichment of ores using a selective scheme to obtain several concentrates.
Zinc concentrates for selective flotation enrichment of polymetallic ores contain, %: Zn – 48...60; Pb – 1.5…2.5; Cu – 1…3; Cd – up to 0.25; Fe – 3…10; S – 30…38, waste rock – up to 10.
Zinc concentrates are complex, expensive raw materials. From them it is necessary to extract zinc, lead, copper, cadmium, sulfur, gold, silver, mercury, gallium, indium, thallium, selenium, tellurium, etc.
Sometimes, when beneficiating difficult-to-enrich copper-zinc ores, intermediate products containing 12...18% Zn and 4...8% Cu are obtained. Recycling of these materials is difficult in both zinc and copper plants.
The processing of zinc concentrates is currently carried out by two methods - pyrometallurgical and hydrometallurgical.
The pyrometallurgical method is based on the process of reduction of zinc oxide at 1000...1100 ºС, i.e. at a temperature above the boiling point of metallic zinc, which ensures its release at the moment of formation in a vaporous state and sublimation in the form of vapors:
ZnO + C = Znsteam + CO; ZnO + CO = Znsteam + CO2.
The zinc vapor subsequently condenses. Obtaining liquid zinc by distillation is only possible under conditions of a highly reducing atmosphere and complete sealing of the equipment used.
Due to the fact that zinc concentrate is a sulfide material, and the recovery of zinc is possible only from its oxide, distillation is preceded by oxidative roasting with complete removal of sulfur.
There are several options for the hardware design of the pyrometallurgical method for producing zinc: in horizontal and vertical retorts, in shaft and electric furnaces. The principle of operation is the basis for the production of zinc vapor in the electrothermal part of the kivtset unit.
The zinc obtained by the pyrometallurgical method necessarily contains a large amount of impurity metals sublimated with it or entering it from dust carried by gases. Therefore, distillation zinc, like any rough metal, needs refining.
The pyrometallurgical method has been used since the emergence of zinc production. The share of zinc production by this method is decreasing from year to year and currently amounts to no more than 20%.
The hydrometallurgical method is currently the main one. The widespread use of hydrometallurgy in the production of zinc is due to its significant advantages over distillation. These include:
- extraction of more zinc and related elements;
- greater complexity of the use of raw materials;
- high quality zinc;
- high mechanization of labor-intensive processes.
Using this method, zinc is leached with a solution of sulfuric acid from a pre-calcined concentrate. During leaching, zinc goes into solution in the form of zinc sulfate by the reaction
ZnO + H2SO4 = ZnSO4 + H2O.
When zinc cinder is leached, the components contained in it partially pass into the solution. The quality of zinc obtained by electrolytic deposition depends on the purity of the solution: the purer the solution entering the electrolysis, the purer the commercial zinc obtained. Therefore, before electrolysis, the solution is thoroughly cleaned of impurities.
The process of electrolytic deposition of zinc from a purified solution proceeds according to the following overall reaction:
ZnSO4 + H2O = Zn + H2SO4 + 0.5O2
During electrolysis, zinc is deposited on the cathode, and sulfuric acid, necessary for leaching fresh portions of cinder, is regenerated at the anode, and oxygen is released. Zinc cathode deposits are melted down and poured into ingots.
Cake (undissolved sediment) obtained after leaching is subjected to additional processing in order to extract zinc and other valuable components from it.
Mixtures and alloys
To enhance strength and increase the melting point, the metal is mixed with copper, aluminum, tin, magnesium and lead.
The most famous and sought after alloy is brass. This is a mixture of copper with the addition of zinc, sometimes tin, nickel, manganese, iron, and lead are also found. The density of brass reaches 8700 kg/m3 . The temperature required for melting is kept at around 880 C o - 950 C o: the higher the zinc content in it, the lower it is. The alloy perfectly resists unfavorable external environments, although it turns black in air if not varnished, it is perfectly polished and welded by resistance welding.
There are two types of brass:
- Alpha brass: more ductile, bends well in any condition, but wears out more.
- Alpha+beta brass: deforms only when heated, but is more wear-resistant. Often alloyed with magnesium, aluminum, lead and iron. This increases strength, but reduces ductility.
Zamak or Zamac alloy is composed of zinc, aluminum, copper and magnesium . The name itself is formed from the first letters of the Latin names: Zink - Aluminum - Magnesium - Kupfer / Cuprum (Zinc-Aluminum-Magnesium-Copper). In the USSR, the alloy was known as TsAM: Zinc-Aluminum-Copper. Actively used in injection molding, melting begins at low temperatures (381 C o - 387 C o) and has a low coefficient of friction (0.07). It has increased strength, which makes it possible to produce products of complex shapes that are not afraid of breaking: door handles, golf clubs, firearms, construction fittings, fasteners of various types and fishing tackle.
A small percentage of zinc (no more than 0.01%) is contained in hart alloys used in printing for casting typographic fonts and rulers, printing forms and typesetting. These are outdated mixtures, replaced by pure zinc with a small addition of impurities.
The low temperature required to melt zinc is often compensated for by alloys with other metals, but it also happens vice versa. If the temperature required to melt a “pure” metal is 419.5 C o , then the alloy with tin is reduced to 199 C o, and with tin and lead - to 150 C o. And although such alloys can be soldered and welded, most often mixtures with zinc are used only to seal existing defects due to their weak strength. For example, an alloy of tin, lead and zinc is recommended for use only on nickel-plated products.
Most often, zinc alloys are used to create carburetors, speedometer frames, radiator grilles, hydraulic brakes, pumps and decorative elements, parts for washing machines, mixers and kitchen equipment, watch cases, typewriters, cash registers and household appliances. These parts cannot be used in industrial production: when the temperature rises to 100 C, the strength of the product decreases by a third, and hardness by almost 40%. When the temperature drops to 0 C, zinc becomes too brittle, which can lead to breakage.
Melting technology
The main part of the charge usually consists of zinc foundry alloys in pigs, return and scrap of zinc alloys. A mixture of calcium, potassium and sodium chlorides, ammonium chloride or cryolite are used as coating fluxes. For blending, primary aluminum in pigs, cathode copper and metallic magnesium are used. All components of the charge must be cleaned of oils, moisture and other impurities. Melting is carried out without allowing the bath to overheat above 480 °C. Based on the results of the express analysis, the chemical composition is adjusted. A steel bell is used to introduce magnesium. Once the desired chemical composition is obtained, the metal is overheated to 440...450°C and poured into a ladle heated to the same temperature. In a ladle under an exhaust hood, the melt is refined using tablets of the complex degasser “Degaser”, which contain 87% hexachloroethane, 12.7% NaCl, 0.3% ultramarine. Refining can also be carried out by settling, purging with inert gases and filtration.
Application
Zinc is one of the most popular metals in the world: it is in third place in terms of production among non-ferrous metals, second only to copper and aluminum. This is also facilitated by its low price. It is most often used for corrosion protection and as part of an alloy such as brass.
- In metallurgy, zinc is especially valuable. It is applied in a thin layer to the steel surface of many metal structures to completely protect them from rust on a mechanical and chemical level. Up to 40% of all production is spent on this. Since zinc, unlike nickel, cobalt, tin and cadmium, is more active than iron, it is the first to come into contact with an unfriendly external environment, completely protecting the base.
- Pure metal is used to recover precious metals after mining by leaching. It is also used to extract gold and silver from rough lead.
- Zinc is the most electropositive metal and practically does not react to water. This made it possible to create a large number of different chemical current sources: zinc-air, silver-zinc, mercury-zinc, “dry” Leclanchet elements.
- Zinc dust is used in fireworks and pyrotechnics to create blue fire, and in paint, especially zinc white, for anti-corrosion protection and better adhesion to the substrate. It is also used to displace precious metals from cyanide solutions and to purify zinc sulfate solutions from cadmium and copper.
- In printing, zinc is used for casting fonts and printing illustrations: zincography has been used since the 19th century. In this case, the printing plate is prepared on a zinc base with a small - no more than 5% - addition of other metals. Before each etching, the plate is annealed and rolled in a heated state.
- In medicine, zinc oxide is used as an antiseptic in the ointments “Lassara Paste”, “Sudocrem”, “Zinc Ointment”, as well as as a powder, toothpastes and material for cementing teeth. Metal is used to create bactericidal ceilings and self-cleaning surfaces. Previously, zinc was used for photocatalytic water purification on an industrial scale.
In living organisms
The human body contains about 2 grams of zinc , and about 400 enzymes contain it. The latter include enzymes that catalyze the hydrolysis of proteins, esters and leptids, the polymerization of RNA and DNA, and the formation of aldehydes. The pure element is found in muscles, pancreas and liver. Men require 11 mg of zinc per day, women - 8 mg.
In the body, zinc performs the following functions:
- Normalizes prostate activity;
- Promotes vitamin E metabolism;
- Takes part in the synthesis of anabolic hormones: growth hormone, insulin, testosterone and others;
- Participates in the production of male hormones and sperm;
- Helps break down alcohol in the body.
With a deficiency of the element in the body, rapid fatigue, irritability , memory loss, loss of vision and weight without an objective reason, allergy attacks, and depression are observed. There is a decrease in insulin levels and accumulation of certain elements in the body: iron, lead, copper, cadmium.
In food
The element is found in meat, cheese, sesame seeds, oysters, chocolate, legumes, oatmeal, sunflower and pumpkin seeds, and is often present in mineral water. The highest percentage of zinc is found in the following products (per 100 grams):
- Oysters (up to 40 mg), anchovies (1.72 mg), octopus (1.68 mg), carp (1.48 mg), caviar (up to 1 mg), herring (about 1 mg).
- Pumpkin seeds (10 mg), sesame seeds (7 mg), sunflower seeds (5.3 mg), peanuts (4 mg), walnuts (3 mg), almonds (3 mg).
- Beef (up to 8.4 mg), lamb (up to 6 mg), beef liver (4 mg), pork (up to 3.5 mg), chicken (up to 3.5 mg).
- Cocoa powder without sugar and sweeteners (6.81 mg), pure dark chocolate (2.3 mg), chocolate candies (up to 2 mg depending on the amount and type of chocolate).
- Lentils (4.78 mg), oats (3.97 mg), wheat (3.46 mg), soybeans (3 mg), rye (2.65 mg), bread (up to 1.5 mg), green peas (1.24 mg), peas (1.2 mg), bamboo shoots (1.1 mg), rice (1 mg), cereal cookies (up to 1 mg).
- Hard cheese (up to 4 mg).