Steel is an alloy of iron mixed with carbon. Its main benefit in construction is strength, because this substance retains its volume and shape for a long time. The whole point is that the particles of the body are in a position of equilibrium. In this case, the attractive and repulsive forces between the particles are equal. The particles are in a clearly defined order.
- Melting temperatures of steel
- Stainless steel
- Cast iron and steel
There are four types of this material: regular, alloy, low-alloy, high-alloy steel. They differ in the number of additives in their composition. The usual one contains a small amount, and then increases. The following additives are used:
- Manganese.
- Nickel.
- Chromium.
- Vanadium.
- Molybdenum.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field . The impact is approximately the same.
When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
This is interesting: Classification and marking of steel
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
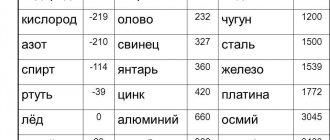
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals . Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Alloy types
Depending on the intensity of heating required for the transition of a metal from one state to another, alloys are divided into several types.
Low fusible. They can be processed even without special equipment. The melting point of steel in degrees Celsius is 600. Low-melting metals include lead, tin and zinc.
Mercury deserves special attention because it can turn into a liquid state at -39°C.
Medium melting. The melting point of steel is in the range of 600°C-1600°C. This category includes aluminum, copper, tin, some types of stainless steel and various alloys with a small chromium content. Medium-melting compounds are most widespread in industry and in everyday life.
Refractory. Compounds included in this category are capable of transitioning from solid to liquid when heated above 1600°C. These are highly alloyed metals, which include tungsten, titanium and chromium. Thanks to these additives, the metal acquires increased strength, resistance to corrosion and chemical influences. In particular, stainless steel is a refractory alloy.
Metal separation
Depending on their melting point, metals are divided into:
- Low-melting: they need no more than 600C
O. This is zinc, lead, hang, tin.
- Medium melting: melting point ranges from 600C
Oup to 1600С
O
. These are gold, copper, aluminum, magnesium, iron, nickel and more than half of all elements.
- Refractory: requires temperatures above 1600C
Oto make the metal liquid. These include chromium, tungsten, molybdenum, titanium.
Depending on the melting temperature, the melting apparatus is also selected . The higher the indicator, the stronger it should be. You can find out the temperature of the element you need from the table.
Another important quantity is the boiling point. This is the value at which the process of boiling liquids begins; it corresponds to the temperature of saturated steam that forms above the flat surface of the boiling liquid. It is usually almost twice the melting point.
Both values are usually given at normal pressure. directly proportional to each other .
- As the pressure increases, the amount of melting increases.
- As the pressure decreases, the amount of melting decreases.
Table of low-melting metals and alloys (up to 600C
O
)
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tin | Sn | 232 C about | 2600 C about |
| Lead | Pb | 327 C about | 1750 C about |
| Zinc | Zn | 420 C o | 907 C o |
| Potassium | K | 63.6 C o | 759 C o |
| Sodium | Na | 97.8 C o | 883 C about |
| Mercury | Hg | — 38.9 C o | 356.73 C o |
| Cesium | Cs | 28.4 C o | 667.5 C o |
| Bismuth | Bi | 271.4 C o | 1564 C about |
| Palladium | Pd | 327.5 C o | 1749 C about |
| Polonium | Po | 254 C about | 962 C about |
| Cadmium | Cd | 321.07 C o | 767 C about |
| Rubidium | Rb | 39.3 C o | 688 C about |
| Gallium | Ga | 29.76 C o | 2204 C about |
| Indium | In | 156.6 C o | 2072 C about |
| Thallium | Tl | 304 C about | 1473 C about |
| Lithium | Li | 18.05 C o | 1342 C about |
Table of medium-melting metals and alloys (from 600C
O
up to 1600С
O
)
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Aluminum | Al | 660 C o | 2519 C about |
| Germanium | Ge | 937 C about | 2830 C about |
| Magnesium | Mg | 650 C o | 1100 C about |
| Silver | Ag | 960 C o | 2180 C about |
| Gold | Au | 1063 C about | 2660 C about |
| Copper | Cu | 1083 C about | 2580 C about |
| Iron | Fe | 1539 C about | 2900 C about |
| Silicon | Si | 1415 C about | 2350 C about |
| Nickel | Ni | 1455 C about | 2913 C about |
| Barium | Ba | 727 C about | 1897 C about |
| Beryllium | Be | 1287 C about | 2471 C about |
| Neptunium | Np | 644 C about | 3901.85 C o |
| Protactinium | Pa | 1572 C about | 4027 C o |
| Plutonium | Pu | 640 C o | 3228 C about |
| Actinium | Ac | 1051 C about | 3198 C about |
| Calcium | Ca | 842 C about | 1484 C about |
| Radium | Ra | 700 C o | 1736.85 C o |
| Cobalt | Co | 1495 C about | 2927 C about |
| Antimony | Sb | 630.63 C o | 1587 C about |
| Strontium | Sr | 777 C about | 1382 C about |
| Uranus | U | 1135 C about | 4131 C about |
| Manganese | Mn | 1246 C about | 2061 C about |
| Konstantin | 1260 C about | ||
| Duralumin | Alloy of aluminum, magnesium, copper and manganese | 650 C o | |
| Invar | Nickel iron alloy | 1425 C about | |
| Brass | Copper and zinc alloy | 1000 C o | |
| Nickel silver | Alloy of copper, zinc and nickel | 1100 C about | |
| Nichrome | Alloy of nickel, chromium, silicon, iron, manganese and aluminum | 1400 C about | |
| Steel | Iron-carbon alloy | 1300 C O — 1500 C O | |
| Fechral | Alloy of chromium, iron, aluminum, manganese and silicon | 1460 C about | |
| Cast iron | Iron-carbon alloy | 1100 C O — 1300 C O | |
Table of refractory metals and alloys (over 1600C
O
)
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tungsten | W | 3420 C about | 5555 C about |
| Titanium | Ti | 1680 C about | 3300 C about |
| Iridium | Ir | 2447 C about | 4428 C about |
| Osmium | Os | 3054 C about | 5012 C about |
| Platinum | Pt | 1769.3 C o | 3825 C about |
| Rhenium | Re | 3186 C about | 5596 C about |
| Chromium | Cr | 1907 C o | 2671 C about |
| Rhodium | Rh | 1964 C about | 3695 C about |
| Ruthenium | Ru | 2334 C about | 4150 C about |
| Hafnium | Hf | 2233 C about | 4603 C about |
| Tantalum | Ta | 3017 C about | 5458 C about |
| Technetium | Tc | 2157 C about | 4265 C about |
| Thorium | Th | 1750 C about | 4788 C about |
| Vanadium | V | 1910 C about | 3407 C about |
| Zirconium | Zr | 1855 C about | 4409 C about |
| Niobium | Nb | 2477 C about | 4744 C about |
| Molybdenum | Mo | 2623 C about | 4639 C about |
| Hafnium carbides | 3890 C about | ||
| Niobium carbides | 3760 C about | ||
| Titanium carbides | 3150 C about | ||
| Zirconium carbides | 3530 C about | ||
General description of the process
To understand at what temperature steel melts, you need to look at this process in more detail. Melting occurs when heated. The material can be heated both from the outside and from the inside. External heating is carried out in thermal furnaces. Resistive heating is used to melt the alloy from the inside. The principle of resistive heating is the electrical resistance that any material has.
Regardless of the type of thermal effect, the same changes occur in materials. Due to heating, thermal vibrations of molecules are enhanced, which leads to structural defects in the lattice. Such changes contribute to the rupture of interatomic bonds, as a result of which the alloy passes into a liquid state.
What is the problem
Designers take into account the melting point of stainless alloys when designing production facilities associated with high temperatures and exposure to aggressive environments. The operating t of the metal, of course, is significantly below the eutectic point (phase transition to the liquid state). The melting point is also the crystallization point; this indicator is important when sterilizing secondary metal and isolating individual components.
When welding metal structures, it is also useful to know at what t a melt pool is formed under the influence of the arc. Heating can affect the condition of the workpieces, leading to internal stresses.
An important factor that influences the eutectic point of stainless alloys is the carbon concentration. The higher the % content of an element, the lower the melting point will be. As the doping fraction increases, the phase transition point depends on the composition and ratio of alloying components. Iron in its pure form belongs to the category of fusible metals; it melts at a temperature higher than alloyed stainless steels. Components that improve the consumer properties of stainless steel belong to various groups:
- fusible (sodium, potassium, bismuth, tin and others);
- medium-melting (main - aluminum, copper, silicon, cobalt);
- refractory (for example, tungsten, titanium, vanadium).
For high-temperature technologies, designers choose stainless alloys with specified physical characteristics. The melting point remains the most important. Sometimes the metal heats up to a critical point. Difficulties in determining the indicator arise due to the multicomponent nature of stainless steel. Depending on the content of alloying components, the metal melts at +1300...1500°C, a spread of 200 degrees is too great to ignore. Carbon steels are cooked at a temperature of +1600°C, but for certain brands of stainless steel such heating will be disastrous.
Melting tables of metals and alloys
Below are tables for a visual acquaintance with the melting temperatures of certain metals and their alloys.
Melting point table for low-melting metals and alloys
Table with melting points of fusible metals
| Name | Designation | Melting | Boiling |
| Tin | Sn | 232°C | 2600°C |
| Lead | Pb | 327°C | 1750°C |
| Zinc | Zn | 420°C | 907°C |
| Potassium | K | 63.6°C | 759°C |
| Sodium | Na | 97.8°C | 883°C |
| Mercury | Hg | -38.9°C | 356.73°C |
| Cesium | Cs | 28.4°C | 667.5°C |
| Bismuth | Bi | 271.4°C | 1564°C |
| Polonium | Po | 254°C | 962°C |
| Cadmium | Cd | 321.07°C | 767°C |
| Rubidium | Rb | 39.3°C | 688°C |
| Gallium | Ga | 29.76°C | 2204°C |
| Indium | In | 156.6°C | 2072°C |
| Thallium | Tl | 304°C | 1473°C |
| Lithium | Li | 18.05°C | 1342°C |
Powered by Inline
Stainless steel
Stainless steel is one of the many iron alloys found in steel. It contains Chromium from 15 to 30%, which makes it rust-resistant, creating a protective layer of oxide on the surface, and carbon. The most popular brands of this type are foreign. These are the 300th and 400th series. They are distinguished by their strength, resistance to adverse conditions and ductility. The 200 series is of lower quality, but cheaper. This is a beneficial factor for the manufacturer. Its composition was first noticed in 1913 by Harry Brearley, who conducted many different experiments on steel.
At the moment, stainless steel is divided into three groups:
- Heat-resistant - at high temperatures it has high mechanical strength and stability. The parts that are made from it are used in the pharmaceutical, rocketry, and textile industries.
- Rust-resistant - has great resistance to rusting processes. It is used in household and medical devices, as well as in mechanical engineering for the manufacture of parts.
- Heat-resistant - resistant to corrosion at high temperatures, suitable for use in chemical plants.
The melting point of stainless steel varies depending on its grade and the number of alloys from approximately 1300 °C to 1400 °C.
Definition of steel: what is it?
Steel is an alloy of iron and carbon , complete with various other elements. At the same time, it must contain at least 45% iron. Since we are talking about composition, let’s consider the classification according to the chemical component.
The main division is into carbon steel and alloy steel (for example, stainless steel). The first type has several subspecies according to the percentage of carbon content:
- low-carbon steels containing up to 0.25% C;
- medium carbon (up to 0.55% C);
- high carbon (from 0.6% to 2% C).
Similarly, the second type is divided into three subtypes according to the content of alloying elements:
- low alloy (up to 4%);
- average (up to 11%);
- highly alloyed (more than 11%).
In addition, steel may contain non-metallic inclusions. Depending on them, they are classified according to another parameter - quality. The lower the percentage of non-metallic inclusions, the higher the quality of the steel. In general, there are four types:
- ordinary;
- quality;
- high quality;
- especially high quality steel.
Its composition also determines the division into types according to purpose. There are many of them, for example, cryogenic steels, structural steels, heat-resistant steels , stainless steels, tool steels, etc. The division into types is also based on structure:
- ferritic;
- austenitic;
- bainitic;
- martensitic;
- pearlite
Two or even more phases may predominate in the structure. In this case, steel is divided into two-phase and multi-phase, respectively.