Melting point of pure aluminum
Melting of aluminum , like other substances, occurs when thermal energy is supplied to it, from the outside or directly into its volume, as happens, for example, with induction heating.
The melting point of aluminum depends on its purity:
- Melting point of ultra-pure aluminum 99.996%: 660.37 °C.
- With an aluminum content of 99.5%, melting begins at 657 °C.
- With an aluminum content of 99.0%, melting begins at 643 °C.
APPLICATION
Aluminum decoration
Widely used as a construction material. The main advantages of aluminum in this quality are lightness, malleability for stamping, and corrosion resistance. The electrical conductivity of aluminum is only 1.7 times less than that of copper, while aluminum is approximately 4 times cheaper per kilogram, but due to its 3.3 times lower density, to obtain equal resistance it needs approximately 2 times less weight . Therefore, it is widely used in electrical engineering for the manufacture of wires, their shielding, and even in microelectronics when depositing conductors on the surface of microcircuit crystals. When aluminum was very expensive, a variety of jewelry was made from it. Thus, Napoleon III ordered aluminum buttons, and in 1889 Mendeleev was presented with scales with bowls made of gold and aluminum. The fashion for jewelry made of aluminum immediately passed when new technologies for its production appeared, reducing the cost many times over. Nowadays, aluminum is sometimes used in the production of costume jewelry.
Read also: Which is better, a hammer drill or an impact drill?
Melting point of metals
Metals and non-metals
Any piece of metal, such as aluminum, contains millions of individual crystals called grains. Each grain has its own unique orientation of the atomic lattice, but collectively the grains are oriented randomly within that piece. This structure is called polycrystalline.
Amorphous materials such as glass differ from crystalline materials such as aluminum in two important ways that are related to each other:
- lack of long-range order in molecular structure
- differences in the nature of melting and thermal expansion.
The difference in molecular structure can be seen in Figure 1. The close-packed and ordered crystal structure is shown on the left. An amorphous material is shown on the right: a less dense structure with a random arrangement of atoms.
Figure 1 – Structure of crystalline (a) and amorphous (b) materials. Crystalline structure: ordered, repeating and dense, amorphous structure - more loosely packed with a random arrangement of atoms.
Melting metals
This difference in structure manifests itself during the melting of metals, including the melting of aluminum of various purities and its alloys. Less densely packed atoms produce an increase in volume (decrease in density) compared to the same metal in a solid crystalline state.
When metals melt, they experience an increase in volume. In pure metals, this volumetric change occurs very sharply and at a constant temperature - the melting point, as shown in Figure 2. This change is a discontinuity between the inclined lines on either side of the melting point. Both of these inclined lines characterize the thermal expansion of the metal, which is usually different in the liquid and solid states.
Figure 2 – Characteristic change in the volume of pure metal compared to the change in the volume of an amorphous material [4]: Tg – glass transition temperature (transition of liquid to solid state); Tm – melting temperature
Heat of Melting
Associated with this sharp increase in volume during the transition of a metal from a solid to a liquid state is a certain amount of heat, which is called the latent heat of fusion. This heat causes the atoms to lose their dense and ordered crystalline structure. This process is reversible, it works in both directions - both during heating and during cooling.
Equilibrium melting point
As shown above, pure crystalline substances, such as pure metals, have a characteristic melting point, often called the “melting point.” At this temperature, this pure crystalline solid melts and becomes a liquid. The transition between solid and liquid states for small samples of pure metals is so small that it can be measured with an accuracy of 0.1 ºC.
Liquids have a characteristic temperature at which they turn into a solid. This temperature is called the solidification temperature or solidification point. Theoretically - under equilibrium conditions - the equilibrium melting temperature of a solid is the same as the equilibrium solidification temperature. In practice, small differences between these values can be observed (Figure 3).
Figure 3 – Cooling and heating curves of pure metal. The phenomena of hypothermia when cooling and overheating when heating are visible. At the beginning of solidification, a depression is observed in the cooling curve, which is explained by the delayed onset of crystallization [4]
Liquidus and solidus temperatures
- The temperature at which melting begins is called the solidus temperature (or solidus point)
- The melting end temperature is the liquidus temperature (or liquidus point).
“Solidus” means, of course, solid, and “liquidus” means liquid: at the solidus temperature the entire alloy is still solid, and at the liquidus temperature it is all already liquid.
When this alloy solidifies from a liquid state, the temperature of the beginning of crystallization (solidification) will be the same liquidus temperature, and the end of crystallization will be the same solidus temperature. At an alloy temperature between its solidus and liquidus temperatures, it is in a semi-liquid-semi-solid, mushy state.
Melting start and end temperatures
These two quantities also need to be taken into account when considering the issue of melting metals:
- the first, “solidus” (solid) is the temperature value upon reaching which the melting process begins;
- the second, “liquidus” (liquid) - indicates the indicator, the achievement of which leads to the completion of melting.
Aluminum-based alloys begin to crystallize when they reach a value called “liquidus.” Curing ends when “solidus” is reached. Between these values, the metal is in a mushy state.
Melting aluminum
Influence of alloying elements and impurities
Adding other elements to aluminum, including alloying ones, reduces its melting point, or more precisely, the beginning of its melting. Thus, for some cast aluminum alloys with a high content of silicon and magnesium, the melting point decreases to almost 500 °C. In general, the concept of “melting point” applies only to pure metals and other crystalline substances. Alloys do not have a specific melting point: the process of their melting (and solidification) occurs in a certain temperature range.
Figure 4 - Change in the specific volume of pure metal (aluminum) and the alloy of this metal (aluminum alloy) [4]
Melting temperature ranges
The table below presents the liquidus and solidus temperatures of some commercial wrought alloys. It must be borne in mind that the concepts of solidus and liquidus temperatures are defined for equilibrium transformations of the liquid phase into the solid phase and vice versa, that is, for infinite duration of the processes. In practice, corrections must be made taking into account the rate of heating or cooling.
Melting silumin
Not all alloys have a range between solidus and liquidus temperatures. Such alloys are called eutectic. For example, in an aluminum alloy containing 12.5% silicon, the liquidus and solidus points are reduced to a point: this alloy, like pure metals, has not an interval, but a melting point. This point and temperature is called eutectic. This alloy belongs to the famous cast aluminum-silicon alloys - silumins with a narrow solidus-liquidus interval, which gives them the best casting properties.
In the Al-Si binary alloy, the solidus temperature is constant and amounts to 577 °C. As the silicon content increases, the liquidus temperature decreases from a maximum value for pure aluminum of 660 °C to coincide with the solidus temperature of 577 °C at a silicon content of 12.6%.
Among other alloying elements of aluminum, magnesium lowers the melting point the most: the eutectic temperature of 450 ° C is achieved with a magnesium content of 18.9%. Copper gives a eutectic temperature of 548 °C, and manganese - only 658 °C! Most alloys are not double, but triple and even quadruple. Therefore, with the combined influence of several alloying elements, the solidus temperature—the beginning of melting or the end of solidification—can be even lower.
At what temperature does aluminum melt?
Aluminum came into industrial and household use relatively recently. At the intersection of the 19th and 20th centuries, the production of this metal on an industrial scale was mastered.
The thing is that the production of many goods began in which aluminum was widely used, for example, in the construction of boats, railway cars, etc.
By the way, it was then that a car with a body made of aluminum was shown to the general public.
Anodized aluminum
Composition and structure of aluminum
Aluminum is the most common metal in the earth's crust. It is classified as a light metal. It has low density and mass. In addition, it has a fairly low melting point. At the same time, it has high ductility and shows good thermal and electrical conductivity characteristics.
Aluminum crystal latticeAluminum structure
The tensile strength of pure aluminum is only 90 MPa. But, if you add some substances to the melt, for example, copper and a number of others, then the tensile strength increases sharply to 700 MPa. The same result can be achieved using heat treatment.
Aluminum, which has extremely high purity - 99.99%, is produced for use in laboratory purposes. For industrial applications, commercially pure aluminum is used.
When producing aluminum alloys, additives such as iron and silicon are used.
They do not dissolve in the aluminum melt, and the additive reduces the ductility of the base material, but at the same time increases its strength.
Appearance of a simple substance
The structure of this metal consists of simple cells consisting of four atoms. This structure is called face-centric.
Calculations show that the density of pure metal is 2.7 kg per cubic meter.
Properties and characteristics
Aluminum is a metal with a silvery-white surface. As already noted, its density is 2.7 kg/m3. The temperature is 660°C.
Its electrical conductivity is equal to 65% of copper and its alloys. Aluminum and most of its alloys are resistant to corrosion. This is due to the fact that an oxide film forms on its surface, which protects the base material from exposure to atmospheric air.
In the untreated state, its strength is 60 MPa, but after adding certain additives it increases to 700 MPa. The hardness in this state reaches 250 HB.
Aluminum can be easily processed under pressure. To remove work hardening and restore ductility after processing, aluminum parts are annealed, and the temperature should be within 350°C.
Melting point of aluminum
The production of aluminum melt, like many other materials, occurs after thermal energy has been supplied to the original metal. It can be supplied either directly into it or from outside.
The melting point of aluminum directly depends on the level of its purity:
- Ultra-pure aluminum melts at a temperature of 660.3°C.
- With an aluminum content of 99.5%, the melting point is 657°C.
- With a content of this metal of 99%, the melt can be obtained at 643°C.
Aluminum meltAluminum production process
An aluminum alloy can contain various substances, including alloying ones. Their presence leads to a decrease in the melting point.
For example, if there is a large amount of silicon, the temperature can drop to 500°C. In fact, the concept of melting point applies to pure metals.
Alloys do not have any constant melting point. This process occurs within a certain heating range.
In materials science there is a concept - solidus and liquidus temperatures.
The first temperature indicates the point at which the melting of aluminum begins, and the second shows at what temperature the alloy will finally melt. In the interval between them, the alloy will be in a mushy state.
Decrease temperature
Before you start melting metal, you can perform certain operations that will reduce the melting temperature. For example, sometimes aluminum powder is melted. In a powdered state, the metal begins to melt somewhat faster.
But with such processing, there is a real danger that when interacting with oxygen contained in the atmosphere, aluminum powder will begin to oxidize with a large release of heat and the formation of metal oxides; this process occurs at a temperature of 2300 degrees.
The main thing is to prevent contact between the melt and water at this moment of melting. This will cause an explosion.
Melting process at home
The relatively low melting point of aluminum allows this operation to be carried out at home.
It should be noted right away that using a powdered mixture as a raw material in a home workshop is too dangerous. Therefore, either ingots or cut wire are used as raw materials.
If there are no special quality requirements for the future product, then anything made from this metal can be used for melting.
Melting aluminum in a homemade forge
In this case, it is not particularly important whether the raw materials are coated with paint or not. When aluminum melts, all foreign substances will simply burn out and be removed along with the slag.
To obtain a high-quality melting result, it is necessary to use materials called fluxes. They are designed to solve the problem of binding and removing foreign impurities and contaminants from the melt.
Means of protection
A home craftsman who decides to melt aluminum at home should be aware that this is a rather dangerous process. And therefore it is impossible to do without the use of protective equipment.
In particular, gloves, an apron, and goggles should be used. The fact is that the melt temperature is within 600 degrees. Therefore, it makes sense to use the protective equipment that welders use.
Using protective equipment when melting aluminum
By the way, when melting aluminum and using cleaning chemicals, it is necessary to protect the respiratory system from combustion products.
Selecting a mold for casting
When choosing a mold for casting aluminum, a home craftsman must understand for what purpose he is processing aluminum. If the future casting is intended for use as solder, then there is no need to use any special forms. To do this, you can use a metal sheet on which to cool the molten metal.
But if there is a need to obtain even a simple part, then the master must decide on the type of mold for casting.
The mold can be made from plaster. To do this, gypsum in a liquid state is poured into an oil-treated mold. After it begins to harden, a casting model is installed into it. In order for molten metal to be poured into the mold, a sprue must be formed.
To do this, a cylindrical part is placed in the mold. Forms can be detachable or not. The process of making a split mold is complicated by the fact that the model will be in two halves. After hardening, they are separated, the model is removed and connected again. The form is ready to use.
Aluminum casting die
To obtain high-quality castings, it is advisable to use metal molds (moulds), but it is advisable to produce them only in factory conditions.
, please select a piece of text and press Ctrl+Enter.
Physical parameters of aluminum and melting point
The melting point of aluminum characterizes the gradient of transition to the liquid state and determines the physical parameters of the chemical element. The properties of the metal make it possible to use it in various branches of industrial production, and the ability to form stable compounds significantly expands the scope of its use.
The ability to change from solid to liquid determines the physical properties of a metal.
Solidification of aluminum
Pure aluminum
Pure metals, including pure aluminum, have a clear melting point - the melting point. Solidification or “freezing” of pure aluminum also occurs at a constant temperature. When pure liquid aluminum cools, its temperature drops to its solidification temperature and remains at that temperature until all of it (liquid aluminum) has solidified. Figures 5 and 6 show typical cooling curves for pure metal as it transitions from liquid to solid.
Figure 5 – Cooling curve of pure metal (for example, aluminum) [3]
Figure 6 – Solidification of pure aluminum [5]
Aluminium alloy
When an aluminum alloy, which consists of aluminum and an alloying element dissolved in it, for example, silicon or copper, solidifies, the cooling curve of this alloy shows that the beginning of solidification occurs at one temperature, and the end at another temperature (Figure 7).
Figure 7 – Cooling curve of an alloy (for example, aluminum alloy) [3]
Melting aluminum alloys for casting
Melting furnaces of various types are used to heat an aluminum alloy to a liquid temperature at which casting operations can be performed. The thermal energy required to heat a metal to a liquid temperature at which it can be poured into molds consists of the sum of the following components:
- Heat to raise the temperature of the metal to its melting point
- Heat of fusion to change a metal from a solid to a liquid state
- Heat to heat the molten metal to the desired casting temperature
Casting temperature is the temperature of the molten metal at which it is poured into the mold. An important factor here is the difference between the casting temperature and the temperature at which solidification begins. This temperature is the melting point for pure aluminum or the liquidus temperature for an aluminum alloy. This temperature difference is sometimes called superheat. This term can also be applied to the amount of heat that must be removed from the liquid metal between casting and the moment it begins to solidify.
Briefly about the process
Melting aluminum at home is not such a difficult process as it may seem at first. The pieces of metal are heated to the required aluminum melting temperature in a special container.
The resulting melt must be kept in a heated state for some time and the resulting slag must be periodically removed from its surface. After this, pure liquid metal is poured into a special mold, in which it will cool for some time.
The time it takes to melt depends on the furnace itself, or more precisely on the temperature it can provide. If a gas burner is used instead of a furnace, then it should heat the metal from above.
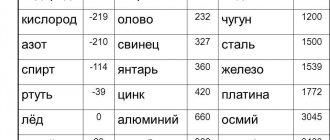
Melting point of various metals
The melting point of some other pure metals is (degrees Celsius)[1]:
- mercury: minus 39
- lithium: 181
- tin: 232
- lead: 328
- zinc: 420
- magnesium: 650
- copper: 1085
- nickel: 1455
- iron: 1538
- titanium: 1670
Sources: 1. Aluminum and Aluminum Alloys, ASM International, 1993 2. Handbook of Aluminum: Vol. 1, ed. GE Totten, DS MacKenzie, 2003 3. Groover, Mikell P. Fundamentals of modern manufacturing: materials, processes and systems, 4th ed. – JOHN WILEY & SONS, 2010 4. Introduction to Alloy Phase Diagrams – ASM International, 1992 5. TALAT 1205
Aluminum
Aluminum is a chemical element of group III of the periodic table of Mendeleev (atomic number 13, atomic mass 26.98154). In most compounds, aluminum is trivalent, but at high temperatures it can also exhibit the +1 oxidation state. Of the compounds of this metal, the most important is Al2O3 oxide.
Aluminum is a silvery-white metal, lightweight (density 2.7 g/cm3), ductile, a good conductor of electricity and heat, melting point 660 °C. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it becomes covered with a protective oxide film - aluminum oxide) and reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide.
- Aluminum dissolves even in dilute hydrochloric and sulfuric acids, especially when heated. But aluminum does not dissolve in highly diluted and concentrated cold nitric acid. When aluminum is exposed to aqueous solutions of alkalis, the oxide layer dissolves, and aluminates are formed - salts containing aluminum as part of the anion:
- Al2O3 + 2NaOH + 3H2O = 2Na[Al(OH)4] .
- Aluminum, devoid of a protective film, interacts with water, displacing hydrogen from it:
- 2Al + 6H2O = 2Al(OH)3 + 3H2
- The resulting aluminum hydroxide reacts with excess alkali, forming hydroxoaluminate:
- Al(OH)3 + NaOH = Na[Al(OH)4].
- The overall equation for the dissolution of aluminum in an aqueous alkali solution has the following form:
- 2Al + 2NaOH +6H2O = 2Na[Al(OH)4] + 3H2.
- Aluminum also actively interacts with halogens. Aluminum hydroxide Al(OH)3 is a white, translucent, gelatinous substance.
- The earth's crust contains 8.8% aluminum. It is the third most abundant element in nature after oxygen and silicon and the first among metals. It is part of clays, feldspars, and mica. Several hundred Al minerals are known (aluminosilicates, bauxites, alunites, and others). The most important aluminum mineral, bauxite, contains 28-60% alumina - aluminum oxide Al2O3.
- Density – 2.7 * 10 3 kg/m 3 ;
- Specific gravity – 2.7 g/cm3;
- Specific heat capacity at 20°C – 0.21 cal/deg;
- Melting point – 658.7°C;
- Specific heat capacity of melting – 76.8 cal/deg;
- Boiling point – 2000°C;
- Relative volume change during melting (ΔV/V) – 6,6%;
- Linear expansion coefficient (at a temperature of about 20°C) : – 22.9 *10 6 (1/deg);
- The thermal conductivity coefficient of aluminum is 180 kcal/m*hour*deg;
Aluminum in its pure form was first obtained by the Danish physicist H. Oersted in 1825, although it is the most common metal in nature. Aluminum production is carried out by electrolysis of alumina Al2O3 in molten cryolite NaAlF4 at a temperature of 950 °C. Aluminum is used in aviation, construction, mainly in the form of aluminum alloys with other metals, electrical engineering (a substitute for copper in the manufacture of cables, etc.), food industry (foil), metallurgy (alloying additive), aluminothermy, etc.
Aluminum density, specific gravity and other characteristics.
Aluminum elastic modulus and Poisson's ratio
| Name of material | Young's modulus, kg/mm 2 | Shear modulus, kg/mm 2 | Poisson's ratio |
| Aluminum bronze, casting | 10500 | 4200 | – |
| Aluminum wire drawn | 7000 | – | – |
| Rolled aluminum | 6900 | 2600-2700 | 0,32-0,36 |
Reflection of light by aluminum
The numbers given in the table show what percentage of light incident perpendicular to the surface is reflected from it.
| Wave name | Wavelength | Light reflection, % |
| Ultraviolet | 1880 2000 2510 3050 3570 | 25 31 53 64 70 |
| Visible | 5000 6000 7000 | – – – |
| Infrared | 8000 10000 50000 100000 | – 74 94 97 |
ALUMINUM OXIDE Al2O3
Aluminum oxide Al2O3 , also called alumina, occurs in nature in crystalline form, forming the mineral corundum. Corundum has very high hardness. Its transparent crystals, colored red or blue, represent the precious stones ruby and sapphire. Currently, rubies are produced artificially by alloying with alumina in an electric furnace. They are used not so much for decoration as for technical purposes, for example, for the manufacture of parts for precision instruments, watch stones, etc. Ruby crystals containing a small admixture of Cr2O3 are used as quantum generators - lasers that create a directed beam of monochromatic radiation.
Read also: Two-key switch Schneider electrician how to connect
Corundum and its fine-grained variety containing a large amount of impurities - emery, are used as abrasive materials.
ALUMINUM PRODUCTION
The main raw material for aluminum production is bauxite containing 32-60% alumina Al2O3. The most important aluminum ores also include alunite and nepheline. Russia has significant reserves of aluminum ore. In addition to bauxite, large deposits of which are located in the Urals and Bashkiria, a rich source of aluminum is nepheline, mined on the Kola Peninsula. A lot of aluminum is also found in deposits in Siberia.
Aluminum is produced from aluminum oxide Al2O3 by the electrolytic method. The aluminum oxide used for this must be sufficiently pure, since impurities are difficult to remove from smelted aluminum. Purified Al2O3 is obtained by processing natural bauxite.
The main starting material for aluminum production is aluminum oxide. It does not conduct electricity and has a very high melting point (about 2050 °C), so it requires too much energy.
It is necessary to reduce the melting point of aluminum oxide to at least 1000 oC. This method was simultaneously found by the Frenchman P. Heroux and the American C. Hall. They found that alumina dissolves well in molten cryolite, a mineral with the composition AlF3.3NaF. This melt is subjected to electrolysis at a temperature of only about 950 °C in aluminum production. Reserves of cryolite in nature are insignificant, so synthetic cryolite was created, which significantly reduced the cost of aluminum production.
A molten mixture of Na3 [AlF6] cryolite and aluminum oxide is subjected to hydrolysis. A mixture containing about 10 weight percent Al2O3 melts at 960 °C and has electrical conductivity, density and viscosity that are most favorable for the process. To further improve these characteristics, AlF3, CaF2 and MgF2 additives are added to the mixture. Thanks to this, electrolysis is possible at 950 °C.
The electrolyser for aluminum smelting is an iron casing lined with refractory bricks on the inside. Its bottom (under), assembled from blocks of compressed coal, serves as a cathode. Anodes (one or more) are located on top: these are aluminum frames filled with coal briquettes. In modern plants, electrolysers are installed in series; each series consists of 150 or more electrolysers.
During electrolysis, aluminum is released at the cathode and oxygen at the anode. Aluminum, which has a higher density than the original melt, is collected at the bottom of the electrolyzer, from where it is periodically released. As the metal is released, new portions of aluminum oxide are added to the melt. The oxygen released during electrolysis interacts with the carbon of the anode, which burns out, forming CO and CO2.
The first aluminum smelter in Russia was built in 1932 in Volkhov.
ALUMINUM ALLOYS
Alloys that increase the strength and other properties of aluminum are obtained by introducing alloying additives into it, such as copper, silicon, magnesium, zinc, and manganese.
Duralumin (duralumin, duralumin, from the name of the German city where industrial production of the alloy began). Aluminum alloy (base) with copper (Cu: 2.2-5.2%), magnesium (Mg: 0.2-2.7%) manganese (Mn: 0.2-1%). Subject to hardening and aging, often clad with aluminum. It is a structural material for aviation and transport engineering.
Silumin - light casting alloys of aluminum (base) with silicon (Si: 4-13%), sometimes up to 23% and some other elements: Cu, Mn, Mg, Zn, Ti, Be). They produce parts of complex configurations, mainly in the automotive and aircraft industries.
Magnalia are alloys of aluminum (base) with magnesium (Mg: 1-13%) and other elements that have high corrosion resistance, good weldability, and high ductility. They produce shaped castings (casting magnalia), sheets, wire, rivets, etc. (deformable magnalia).
The main advantages of all aluminum alloys are their low density (2.5-2.8 g/cm3), high strength (per unit weight), satisfactory resistance to atmospheric corrosion, comparative cheapness and ease of production and processing.
Aluminum alloys are used in rocketry, aircraft, auto, shipbuilding and instrument making, in the production of tableware, sporting goods, furniture, advertising and other industries.
Aluminum alloys occupy second place in terms of the breadth of application after steel and cast iron.
Aluminum is one of the most common additives in alloys based on copper, magnesium, titanium, nickel, zinc, and iron.
Aluminum is also used for aluminizing (aluminizing) - saturating the surface of steel or cast iron products with aluminum in order to protect the base material from oxidation under strong heating, i.e. increasing heat resistance (up to 1100 °C) and resistance to atmospheric corrosion.
Oxidation of liquid aluminum
It may seem that at this stage the most efficient way to complete the melting cycle is to increase the melt temperature. But, unfortunately, aluminum in the liquid state exhibits too high chemical activity.
Figure 8 shows the effect of increasing the temperature of the aluminum melt on the formation of slag (Al2O3). When the temperature of aluminum exceeds 760ºC, the rate of slag formation increases sharply. The more slag is formed, the more metal is irretrievably lost.
For the formation of slag, in addition to high temperature, the presence of oxygen in contact with the metal is necessary. The main sources of oxygen in the furnace volume are air that penetrates from outside and air that has not had time to burn in the burner. A good burner should operate without supplying excess air to the furnace volume.
Figure 8 – Dependence of the rate of aluminum oxidation on temperature [2]
Melt mixing
To increase the heating rate of the melt, various methods of mixing it are used. This is most often done using mechanical tools such as hand scrapers or large scrapers mounted on a forklift. However, within a few minutes after the end of this operation, the melt pool again returns to its previous stable state [1]. In addition, for such mixing it is necessary to open the furnace loading window, which leads to additional formation of slag. Therefore, large furnaces and large industries use complex systems for mixing the melt using various pumps - centrifugal, electromagnetic and others, which can mix the melt in a continuous mode.
Sources:
- Handbook of Aluminum Recycling / Ch. Schmitz, 2006
- Direct Charged Melters / Donald F. Whipple – Bloomengineering, 2004
- Handbook of Aluminum: Vol. 1/ed. [email protected] , 2003
Properties and characteristics
Aluminum is a metal with a silvery-white surface. As already noted, its density is 2.7 kg/m3. The temperature is 660°C.
Its electrical conductivity is equal to 65% of copper and its alloys. Aluminum and most of its alloys are resistant to corrosion. This is due to the fact that an oxide film forms on its surface, which protects the base material from exposure to atmospheric air.
In the untreated state, its strength is 60 MPa, but after adding certain additives it increases to 700 MPa. The hardness in this state reaches 250 HB.
Aluminum can be easily processed under pressure. To remove work hardening and restore ductility after processing, aluminum parts are annealed, and the temperature should be within 350°C.
Read also: How to make a manual hoist
Furnaces for melting aluminum
- companies creating casting alloys for aluminum casting manufacturers
- companies that create aluminum for steel deoxidation.
Both categories of companies use “old” scrap and production waste from foundries as raw materials. At such plants, in addition to introducing alloying components to refine a certain alloy, they use equipment to purify the aluminum melt and eliminate unwanted chemical elements and other impurities. Rotary melting furnaces are used by these aluminum scrap processors. Aluminum melting in foundries that produce aluminum castings from recycled aluminum is carried out mainly in crucible furnaces - gas and electric, induction and resistance, both for melting and holding aluminum, as well as for pouring molten aluminum into prepared molds. The melting point of aluminum oxide is approximately 2050 ° C, which is almost three times higher than the melting point of metallic aluminum. Today, the most popular is the smelting of aluminum in flame reverberatory furnaces, which operate on carbon fuel, and in electric furnaces. During the melting of aluminum in reverberatory flame furnaces and in chamber electric resistance furnaces, heating of individual pieces of the charge starts in the region of the highest temperatures, i.e. in the upper part. At the same time, the surface of the cage oxidizes at high speed and absorbs many gases. Inside a channel induction electric furnace, the melting of pieces of aluminum is carried out in the region of highest temperatures under a layer of liquid metal, the surface of which is covered with a film of aluminum oxide. The region of highest temperatures in channel electric furnaces is located in a narrow channel and in the adjacent parts of the charge. The metal on the surface of the shaft has the lowest temperature, as a result of which the resulting castings from channel electric furnaces contain a lower amount of oxides than castings from other types of furnaces. Crucible induction electric furnaces have the same advantage, in which, according to technological requirements, a certain amount of liquid metal remains in the crucible at the end of each melt, approximately 20-35% of the capacity of the furnace crucible. An important property of liquid aluminum and its alloys is its ability to absorb gases, especially hydrogen.
Composition and structure of aluminum
Aluminum is the most common metal in the earth's crust. It is classified as a light metal. It has low density and mass. In addition, it has a fairly low melting point. At the same time, it has high ductility and shows good thermal and electrical conductivity characteristics.
Aluminum crystal lattice
The tensile strength of pure aluminum is only 90 MPa. But, if you add some substances to the melt, for example, copper and a number of others, then the tensile strength increases sharply to 700 MPa. The same result can be achieved using heat treatment.
Aluminum, which has extremely high purity - 99.99%, is produced for use in laboratory purposes. For industrial applications, commercially pure aluminum is used. When producing aluminum alloys, additives such as iron and silicon are used. They do not dissolve in the aluminum melt, and the additive reduces the ductility of the base material, but at the same time increases its strength.
Appearance of a simple substance
The structure of this metal consists of simple cells consisting of four atoms. This structure is called face-centric.
Calculations show that the density of pure metal is 2.7 kg per cubic meter.
This is interesting: Characteristics and application of U8 steel