Medium melting metals
Medium-melting metals begin to transform from solid to liquid at temperatures from 600°C to 1600°C. They are used to make slabs, reinforcements, blocks and other metal structures suitable for construction. This group of metals includes iron, copper, aluminum, and they are also part of many alloys. Copper is added to alloys of precious metals such as gold, silver, and platinum. 750 gold consists of 25% alloy metals, including copper, which gives it a reddish tint. The melting point of this material is 1084 °C. And aluminum begins to melt at a relatively low temperature of 660 degrees Celsius. This is a lightweight, ductile and inexpensive metal that does not oxidize or rust, therefore it is widely used in the manufacture of tableware. The melting point of iron is 1539 degrees. This is one of the most popular and affordable metals, its use is widespread in the construction and automotive industries. But due to the fact that iron is subject to corrosion, it must be additionally processed and covered with a protective layer of paint, drying oil, or prevent moisture from entering.
Heat of Melting
Now let's move on from the definition of melting to a formula that quantitatively describes this process. When melting occurs, the external heat supply is used to break the bonds in the solid and transform it into a liquid state. The energy that must be expended in order for a certain amount of a solid substance at the melting point to transform into a liquid state is called the heat of fusion. The formula in this case is written as follows: λ=Q/m, where Q is the amount of heat, m is the mass of the body.
The value of the heat of fusion λ depends on the physical and chemical properties of the material. For example, for ice this value is 333.55 J/g or 6.02 kJ/mol, and for iron 13.81 kJ/mol. The values are given at a pressure of 1 atmosphere.
Refractory metals
The temperature of refractory metals is above 1600°C. These are tungsten, titanium, platinum, chromium and others. They are used as light sources, machine parts, lubricants, and in the nuclear industry. They are used to make wires, high-voltage wires, and are used to melt other metals with a lower melting point. Platinum begins to transition from solid to liquid at a temperature of 1769 degrees, and tungsten at a temperature of 3420°C.
Mercury is the only metal that is in a liquid state under normal conditions, namely, normal atmospheric pressure and average ambient temperature. The melting point of mercury is minus 39°C. This metal and its vapors are poisonous, so it is used only in closed containers or in laboratories. A common use of mercury is as a thermometer to measure body temperature.
Density.
This is one of the most important characteristics of metals and alloys. According to their density, metals are divided into the following groups:
lungs
(density not more than 5 g/cm3) – magnesium, aluminum, titanium, etc.:
heavy
– (density from 5 to 10 g/cm3) – iron, nickel, copper, zinc, tin, etc. (this is the most extensive group);
very heavy
(density more than 10 g/cm3) – molybdenum, tungsten, gold, lead, etc.
Table 2 shows the density values of metals. (This and the following tables characterize the properties of those metals that form the basis of alloys for artistic casting).
Table 2. Metal density.
Melting temperature.
Depending on the melting point, the metal is divided into the following groups:
fusible
(melting point does not exceed 600 o C) – zinc, tin, lead, bismuth, etc.;
medium-melting
(from 600 o C to 1600 o C) – these include almost half of the metals, including magnesium, aluminum, iron, nickel, copper, gold;
refractory
(more than 1600 o C) – tungsten, molybdenum, titanium, chromium, etc.
Mercury is a liquid.
When making artistic castings, the melting point of the metal or alloy determines the choice of melting unit and refractory molding material. When additives are introduced into a metal, the melting point, as a rule, decreases.
Table 3. Melting and boiling points of metals.
Specific heat. This is the amount of energy required to raise the temperature of a unit mass by one degree. Specific heat capacity decreases with increasing atomic number of an element in the periodic table. The dependence of the specific heat capacity of an element in the solid state on atomic mass is described approximately by the Dulong and Petit law:
where, ma
– atomic mass;
cm
– specific heat capacity (J/kg * o C).
Table 4 shows the specific heat capacity of some metals.
Table 4. Specific heat capacity of metals.
Latent heat of fusion of metals. This characteristic (Table 5), along with the specific heat capacity of the metals, largely determines the required power of the melting unit. Melting a low-melting metal sometimes requires more thermal energy than a refractory metal. For example, heating copper from 20 to 1133 o C will require one and a half times less thermal energy than heating the same amount of aluminum from 20 to 710 o C.
Table 5. Latent heat of metal
Heat capacity. Heat capacity characterizes the transfer of thermal energy from one part of the body to another, or more precisely, the molecular transfer of heat in a continuous medium due to the presence of a temperature gradient. (Table 6)
Table 6. Thermal conductivity coefficient of metals at 20 o C
The quality of artistic casting is closely related to the thermal conductivity of the metal. During the smelting process, it is important not only to ensure a sufficiently high temperature of the metal, but also to achieve a uniform temperature distribution throughout the entire volume of the liquid bath. The higher the thermal conductivity, the more uniformly the temperature is distributed. During electric arc melting, despite the high thermal conductivity of most metals, the temperature difference across the cross section of the bath reaches 70-80 o C, and for a metal with low thermal conductivity this difference can reach 200 o C or more.
Read also: How to restore threads in an aluminum case
Favorable conditions for temperature equalization are created during induction melting.
Thermal expansion coefficient
. This value, which characterizes the change in the dimensions of a 1 m long sample when heated by 1 o C, is important for enamel work (Table 7)
The thermal expansion coefficients of the metal base and enamel should be as close as possible so that the enamel does not crack after firing. Most enamels representing a solid coefficient of silicon oxides and other elements have a low coefficient of thermal expansion. As practice has shown, enamels adhere very well to iron and gold, and less firmly to copper and silver. It can be assumed that titanium is a very suitable material for enameling.
Table 7. Thermal expansion coefficient of metals.
Reflectivity. This is the ability of a metal to reflect light waves of a certain length, which is perceived by the human eye as color (Table 8). Metal colors are shown in Table 9.
Table 8. Correspondence between color and wavelength.
Table 9. Metal colors.
Pure metals are practically not used in decorative and applied arts. For the manufacture of various products, alloys are used, the color characteristics of which differ significantly from the color of the base metal.
Over the course of a long time, vast experience has been accumulated in the use of various casting alloys for the manufacture of jewelry, household items, sculptures and many other types of artistic casting. However, the relationship between the structure of the alloy and its reflectivity has not yet been revealed.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
Capillary method
The melting point, determined by the capillary method, is the temperature at which the last solid particle of a compacted column of substance in the capillary passes into the liquid phase.
Device 1.
The components of the device are:
- a glass vessel containing a liquid (such as water, petroleum jelly or silicone oil) used as a bath and equipped with a suitable heating device. The liquid in the bath should be selected depending on the required temperature;
- a mixing device that ensures uniform temperature inside the bath;
- a suitable thermometer with a division value of no more than 0.5 °C. The difference between the upper and lower divisions of the thermometer in the area of the measured temperature is no more than 100 °C;
- sealed at one end capillaries made of neutral durable glass with a diameter of 0.9 to 1.1 mm, a wall thickness of 0.10 to 0.15 mm and a length of 10 cm.
Device 2.
The components of the device are:
- round-bottomed heat-resistant glass flask with a capacity of 100 to 150 ml; flask neck length 20 cm; throat diameter – from 3 to 4 cm;
- a heat-resistant glass test tube inserted into the flask and spaced 1.0 cm from the bottom of the flask; tube diameter from 2.0 to 2.5 cm;
- a short mercury glass thermometer with a division value of 0.5°C, inserted into the inner test tube so that its end is 1.0 cm from the bottom of the test tube;
- heating source (gas burner, electric heating);
- sealed at one end capillaries made of neutral durable glass with a diameter of 0.9 to 1.1 mm, a wall thickness of 0.10 to 0.15 mm and a length of 6 to 8 cm.
The flask is filled to ¾ volume with the appropriate liquid:
- Vaseline oil or liquid silicones; concentrated sulfuric acid – for substances with a melting point from 80 to 260 °C;
- a solution of potassium sulfate in concentrated sulfuric acid (3:7 by weight) - for substances with a melting point above 260 °C;
- purified water – for substances with a melting point below 80°C.
Notes.
- The glass tubes from which the capillaries are drawn must be washed and dried.
- When preparing a solution of potassium sulfate in concentrated sulfuric acid, the mixture is boiled for 5 minutes with vigorous stirring. Insufficient mixing may result in the formation of 2 layers, which may result in the mixture boiling, leading to an explosion.
Device 3.
A device for determining the melting temperature with a measurement range from 20 to 360 °C with electrical heating of the PTP type or PTP-M type (Fig. 1) with a measurement range from 20 to 340 °C.
The components of the device are:
- base with control panel and nomogram;
- glass block heater, heating of which is carried out by constantan wire wound bifilarly;
- optical device;
- device for installing a thermometer;
- device for installing capillaries;
- short thermometer with a division value of 0.5 ºС;
- heating source (electric heating);
- capillaries 20 cm long for a PTP type device; capillaries 8 cm long for a PTP-M type device.
The principle of operation of the device is based on the temperature effect on the substances under study in vertically installed capillaries, sealed at the lower end.
The use of other devices using the capillary method is allowed if the accuracy and accuracy of the measurements is no worse than in the case of the use of the devices described above.
PTP-M device for determining melting point
Figure 1 – PTP-M device for determining the melting point
Methodology . Unless otherwise specified in the monograph, the finely powdered substance is dried either at a temperature of 100 to 105 °C for 2 hours, or in a desiccator over sulfuric acid for 24 hours, or in a vacuum over anhydrous silica gel for 24 hours.
A sufficient amount of the substance is placed in the capillary until a compacted column about 5 mm high is obtained. The necessary compaction of the substance when filling the capillary can be obtained by throwing it several times, sealed end down, into a glass tube 0.5 - 1.0 m long, placed vertically on the glass. The capillary with the substance is stored in a desiccator until the determination begins.
Raise the temperature in the bath (device). At a temperature of approximately 10 °C below the expected melting point, adjust the heating of the apparatus so that the rate of temperature rise throughout the test is approximately 1 °C per minute. When the temperature reaches a value 5 - 10 °C below the expected melting point, the capillary with the substance is attached to the thermometer so that its sealed end is at the level of the center of the thermometer ball, and is placed in the device.
Continue heating at the rate:
- for substances that are stable when heated when determining the melting point below 100 °C - at a rate of 0.5 to 1.0 °C per minute;
- when determining the melting temperature from 100 to 150 °C - from 1.0 to 1.5 °C per 1 min;
- when determining the melting temperature above 150 °C - from 1.5 to 2.0 °C per 1 min;
- for substances that are unstable when heated from 2.5 to 3.5 ° C per 1 min.
The temperature at which the last solid particle passes into the liquid phase is noted.
At least two determinations are made. The melting point is taken as the arithmetic mean of several determinations carried out under the same conditions and differing from each other by no more than 1 °C.
Note. When determining the melting point, the flask and test tube must be open.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal purposes, resistive heating is used, passing electric current or induction heating in a high-frequency electromagnetic field
. The impact is approximately the same.
When does heating occur
, the amplitude of thermal vibrations of molecules increases.
Structural lattice defects
appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting – from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory – from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Characteristics table
Metals and alloys are an indispensable basis for forging
, foundry production, jewelry production and many other areas of production.
Whatever the craftsman does ( gold jewelry
, cast iron fences, steel knives or
copper bracelets)
, in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
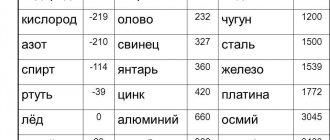
- aluminum – 660 °C;
- copper melting point – 1083 °C;
- gold melting point – 1063 °C;
- silver – 960 °C;
- tin – 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead – 327 °C;
- melting point of iron – 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) – from 1100 °C to 1300 °C;
- mercury – -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum
- 2519 °C
, for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium – 5596
°C
. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals
.
The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium
and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Read also: Marking the ceiling with a laser level
Another indicator found in the tables is the thermal conductivity of metals
. Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Melting point, along with density, refers to the physical characteristics of metals
.
The melting point of a metal
is the temperature at which a metal changes from its normal solid state (except mercury) to a liquid state when heated.
During melting, the volume of the metal practically does not change, so normal atmospheric pressure does not affect
.
The melting point of metals ranges from -39 degrees Celsius to +3410 degrees
. For most metals, the melting point is high, however, some metals can be melted at home by heating on a regular burner (tin, lead).
Classification of metals by melting point
- Low-melting metals
, the melting point of which ranges
up to 600
degrees Celsius, for example
zinc, tin, bismuth
. - Medium-melting metals
that melt at temperatures
from 600 to 1600
degrees Celsius: such as
aluminum, copper, tin, iron
. - Refractory metals
, the melting point of which reaches
more than 1600
degrees Celsius -
tungsten, titanium, chromium
, etc. - - the only metal that is in a liquid state under normal conditions (normal atmospheric pressure, average ambient temperature). The melting point of mercury is about -39 degrees
Celsius.
Table of melting temperatures of metals and alloys
650
1000
| Metal | |
| Aluminum | 660,4 |
| Tungsten | 3420 |
| Duralumin | |
| Iron | 1539 |
| Gold | 1063 |
| Iridium | 2447 |
| Potassium | 63,6 |
| Silicon | 1415 |
| Brass | |
| Low melting alloy | 60,5 |
| Magnesium | 650 |
| Copper | 1084,5 |
| Sodium | 97,8 |
| Nickel | 1455 |
| Tin | 231,9 |
| Platinum | 1769,3 |
| Mercury | –38,9 |
| Lead | 327,4 |
| Silver | 961,9 |
| Steel | 1300-1500 |
| Zinc | 419,5 |
| Cast iron | 1100-1300 |
When melting metal for the manufacture of metal castings, the choice of equipment, material for molding the metal, etc. depends on the melting temperature. It should also be remembered that when alloying the metal with other elements, the melting temperature most often decreases
.
Do not confuse the concepts of “metal melting point” and “metal boiling point” - for many metals these characteristics are significantly different: for example, silver melts at a temperature of 961 degrees Celsius, and boils only when the temperature reaches 2180 degrees.
Melting of a metal is a certain thermodynamic process in which the crystal lattice of the metal is destroyed and it passes from the solid phase state to the liquid.
The melting point of metals is an indicator of the temperature of the heated metal, upon reaching which the process of phase transition (melting) begins. The process itself is the reverse of crystallization and is inextricably linked with it. To melt metal? it must be heated using an external heat source to the melting point, and then continue to supply heat to overcome the phase transition energy. The fact is that the very value of the melting point of metals indicates the temperature at which the material will be in phase equilibrium, at the boundary between a liquid and a solid. At this temperature, pure metal can exist simultaneously in both solid and liquid states. To carry out the melting process, it is necessary to overheat the metal slightly above the equilibrium temperature in order to provide a positive thermodynamic potential. Give a kind of impetus to the process.
The melting point of metals is constant only for pure substances. The presence of impurities will shift the equilibrium potential in one direction or another. This happens because a metal with impurities forms a different crystal lattice, and the forces of interaction between atoms in them will differ from those present in pure materials. Depending on the melting point, metals are divided into low-melting ones (up to 600 ° C, such as gallium , mercury), medium-melting (600-1600°C, copper, aluminum) and refractory (>1600°C, tungsten, molybdenum).
In the modern world, pure metals are rarely used due to the fact that they have a limited range of physical properties. Industry has long and extensively used various combinations of metals - alloys, the varieties and characteristics of which are much greater. The melting point of the metals that make up various alloys will also differ from the melting point of their alloy. Different concentrations of substances determine the order of their melting or crystallization. But there are equilibrium concentrations at which the metals that make up the alloy solidify or melt simultaneously, that is, they behave like a homogeneous material. Such alloys are called eutectic.
Knowing the melting point is very important when working with metal; this value is necessary both in production, for calculating the parameters of alloys, and during the operation of metal products, when the phase transition temperature of the material from which the product is made determines the restrictions on its use. For convenience, these data are summarized in a single table. The metal melting table is a summary result of physical studies of the characteristics of various metals. There are also similar tables for alloys. The melting point of metals also significantly depends on pressure, so these tables are relevant for a specific pressure value (usually these are normal conditions when the pressure is 101.325 kPa). The higher the pressure, the higher the melting point, and vice versa.
In the metallurgical industry, one of the main areas is the casting of metals and their alloys due to the low cost and relative simplicity of the process. Molds with any shape and various dimensions can be cast, from small to large; It is suitable for both mass and customized production.
Casting is one of the oldest areas of working with metals, and begins around the Bronze Age: 7-3 millennium BC. e. Since then, many materials have been discovered, leading to advancements in technology and increased demands on the foundry industry.
Nowadays, there are many directions and types of casting, differing in technological process. One thing remains unchanged - the physical property of metals to pass from a solid to a liquid state, and it is important to know at what temperature the melting of different types of metals and their alloys begins.
Read also: Chainsaw partner 350 where is the breather located
Melting is a first order phase transition
According to its definition, melting is a first-order transition, since it involves the absorption of heat. In this case, the temperature of the entire system does not change during the melting process and is a constant value. This fact is explained by the fact that the heat supplied to the body is spent not on increasing the kinetic energy of atoms and molecules, but on breaking strong chemical bonds between them. Only after all the bonds in the solid have been broken, further heat supply to the already liquid substance will lead to an increase in its temperature.
The melting process itself does not occur spontaneously, but develops over a certain period of time when the liquid and solid phases coexist in equilibrium with each other.
Thus, melting is an endothermic process, which means that it involves the absorption of heat. The reverse process, in which the liquid solidifies, is called crystallization.
Metal melting process
This process refers to the transition of a substance from a solid to a liquid state. When the melting point is reached, the metal can be in either a solid or liquid state; further increase will lead to the complete transition of the material into a liquid.
The same thing happens during solidification - when the melting point is reached, the substance will begin to transition from a liquid to a solid state, and the temperature will not change until complete crystallization.
It should be remembered that this rule applies only to pure metal. Alloys do not have a clear temperature boundary and undergo state transitions in a certain range:
- Solidus is the temperature line at which the most fusible component of the alloy begins to melt.
- Liquidus is the final melting point of all components, below which the first alloy crystals begin to appear.
It is impossible to accurately measure the melting point of such substances; the point of transition of states is indicated by a numerical interval.
Depending on the temperature at which metals begin to melt, they are usually divided into:
- Low-melting, up to 600 °C. These include tin, zinc, lead and others.
- Medium melting, up to 1600 °C. Most common alloys, and metals such as gold, silver, copper, iron, aluminum.
- Refractory, over 1600 °C. Titanium, molybdenum, tungsten, chromium.
There is also a boiling point - the point at which the molten metal begins to transition into a gaseous state. This is a very high temperature, typically 2 times the melting point.
Effect of pressure
The melting temperature and the equal solidification temperature depend on pressure, increasing with its increase. This is due to the fact that with increasing pressure the atoms come closer to each other, and in order to destroy the crystal lattice they need to be moved away. At increased pressure, greater thermal energy is required and the corresponding melting temperature increases.
There are exceptions when the temperature required to transform into a liquid state decreases with increased pressure. Such substances include ice, bismuth, germanium and antimony.
Kinetic and potential energy
To understand what melting is in physics, it is necessary to clearly understand the relationship between kinetic and potential energy in solid and liquid bodies.
Potential energy characterizes the work that must be expended to disperse a given body in space into its constituent particles. To describe this quantity, the concept of binding energy is introduced, which denotes the work required to tear off one atom or molecule from a body and remove it to infinity. For example, typical binding energies for solids are several electron volts; these values for liquids are an order of magnitude lower.
Kinetic energy characterizes the intensity of movement of atoms and molecules. In the case of condensed matter, this energy is directly proportional to temperature.
In solids, the kinetic energy at room temperatures is several hundredths of an electron volt, that is, it is 100 times less than the potential. Atoms and molecules in solids are, as it were, in a potential well and vibrate around certain stable positions. They can get out of these positions if the fluctuations in kinetic energy turn out to be significant, or if the potential well itself is small, for example, when there is some kind of defect nearby.
The kinetic energy of atoms and molecules in a liquid is approximately equal to their potential energy, that is, a few tenths of an electron volt at room temperature. This means that each particle that makes up the liquid is constantly jumping from one place to another. A good proof of this fact is Brownian motion.
Melting point table
It is important for anyone involved in the metallurgical industry, whether a welder, foundry worker, smelter or jeweler, to know the temperatures at which the materials they work with melt. The table below shows the melting points of the most common substances.
Table of melting temperatures of metals and alloys
| Name | T pl, °C |
| Aluminum | 660,4 |
| Copper | 1084,5 |
| Tin | 231,9 |
| Zinc | 419,5 |
| Tungsten | 3420 |
| Nickel | 1455 |
| Silver | 960 |
| Gold | 1064,4 |
| Platinum | 1768 |
| Titanium | 1668 |
| Duralumin | 650 |
| Carbon steel | 1100−1500 |
| Cast iron | 1110−1400 |
| Iron | 1539 |
| Mercury | -38,9 |
| Cupronickel | 1170 |
| Zirconium | 3530 |
| Silicon | 1414 |
| Nichrome | 1400 |
| Bismuth | 271,4 |
| Germanium | 938,2 |
| Tin | 1300−1500 |
| Bronze | 930−1140 |
| Cobalt | 1494 |
| Potassium | 63 |
| Sodium | 93,8 |
| Brass | 1000 |
| Magnesium | 650 |
| Manganese | 1246 |
| Chromium | 2130 |
| Molybdenum | 2890 |
| Lead | 327,4 |
| Beryllium | 1287 |
| Will win | 3150 |
| Fechral | 1460 |
| Antimony | 630,6 |
| titanium carbide | 3150 |
| zirconium carbide | 3530 |
| Gallium | 29,76 |
In addition to the melting table, there are many other supporting materials. For example, the answer to the question what is the boiling point of iron lies in the table of boiling substances. In addition to boiling, metals have a number of other physical properties, such as strength.
Open capillary method
A glass capillary is used, open at both ends, about 80 mm long, with an outer diameter of 1.4 to 1.5 mm and an inner diameter of 1.0 to 1.2 mm.
The substance, previously prepared as specified in the pharmacopoeial monograph, is placed in each of the 5 capillaries in an amount sufficient to form a column about 10 mm high in each capillary. The capillaries are left for a certain time at the temperature specified in the pharmacopoeial monograph.
Attach one of the capillaries to a thermometer with a division value of 0.2 °C so that the substance is located near the thermometer ball.
A thermometer with an attached capillary is placed in a glass so that the distance between the bottom of the glass and the bottom of the thermometer ball is 1 cm. The glass is filled with water to a layer height of 5 cm.
Raise the water temperature at a rate of 1 °C per minute.
The melting point is the temperature at which the substance begins to rise through the capillary. In cases where the column of the substance does not rise in the capillary, the melting point is taken to be the temperature at which the column of the substance in the capillary becomes transparent.
Repeat this operation with 4 other capillaries and calculate the result as the arithmetic mean of 5 values. The discrepancy between all values should not exceed 1 °C.
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Metal strength table
| Metal | Resistance, MPa |
| Copper | 200−250 |
| Silver | 150 |
| Tin | 27 |
| Gold | 120 |
| Lead | 18 |
| Zinc | 120−140 |
| Magnesium | 120−200 |
| Iron | 200−300 |
| Aluminum | 120 |
| Titanium | 580 |
The most common alloys in everyday life
As can be seen from the table, the melting points of elements vary greatly even among materials commonly found in everyday life.
Thus, the minimum melting point of mercury is -38.9 °C, so at room temperature it is already in a liquid state. This explains why household thermometers have a lower mark of -39 degrees Celsius: below this indicator, mercury turns into a solid state.
The most common solders in household use contain a significant percentage of tin, which has a melting point of 231.9 °C, so most solders melt at the operating temperature of the soldering iron 250−400 °C.
In addition, there are low-melting solders with a lower melt limit, up to 30 °C, and are used when overheating of the materials being soldered is dangerous. For these purposes, there are solders with bismuth, and the melting of these materials lies in the range from 29.7 - 120 °C.
Melting of high-carbon materials, depending on alloying components, ranges from 1100 to 1500 °C.
The melting points of metals and their alloys are in a very wide temperature range, from very low temperatures (mercury) to several thousand degrees. Knowledge of these indicators, as well as other physical properties, is very important for people who work in the metallurgical field. For example, knowledge of the temperature at which gold and other metals melt will be useful to jewelers, foundries and smelters.
Drop method
This method determines the temperature at which, under the conditions given below, the first drop of molten test substance falls from the cup.
Device . The device consists of two metal sleeves (A and B) connected by thread. The sleeve (A) is attached to a mercury thermometer. In the lower part of the sleeve (B), a metal cup (D) is loosely fixed using two seals (D). The exact position of the cup is determined by 2 mm long clamps (E), which are also used to center the thermometer. The hole (B) in the wall of the sleeve (B) is intended to equalize pressure. The discharge surface of the cup should be flat, and the edges of the outlet opening should be at right angles to the surface. The lower part of the mercury thermometer has the shape and size as shown in Fig. 2. The thermometer is graduated from 0 to 110 ºС and a distance on the scale of 1 mm corresponds to a temperature difference of 1 ºС. The mercury bulb of the thermometer has a diameter of (3.5 ± 0.2) mm and a height of (6.0 ± 0.3) mm.
The device is installed along the axis of a test tube with a length of about 200 mm and an outer diameter of about 40 mm.
The device is attached to the test tube using a stopper into which a thermometer is inserted and which has a side slot. The opening of the cup should be about 15 mm from the bottom of the tube. The entire device is immersed in a glass with a capacity of about 1 liter filled with water. The bottom of the test tube should be at a distance of about 25 mm from the bottom of the beaker. The water level should reach the top of the sleeve (A). To evenly distribute the temperature in the glass, use a stirrer.
Dropping point determination device
Figure 2
.Dimensions in mm
Methodology. Fill the cup to the brim with the unmelted test substance, unless otherwise indicated in the pharmacopoeial monograph. Excess substance is removed from both sides with a spatula. After connecting the sleeves (A) and (B), push the cup inward into its place in the sleeve (B) until it stops. Remove the substance squeezed out by the thermometer with a spatula. The device is placed in a water bath as described above. The water bath is heated to a temperature approximately 10 ºC below the expected melting point and the heating rate is set to about 1 ºC per minute. The temperature at which the first drop falls is noted. At least three determinations are carried out, each time with a new sample of the substance. The difference between readings should not exceed 3 °C. The arithmetic mean is calculated from the obtained values.
Download in PDF OFS.1.2.1.0011.15 Melting point