Chemical-thermal treatment
Chemical-thermal treatment is a process of chemical and thermal effects on the surface layer of steel in order to change the composition, structure and properties.
Chemical-thermal treatment increases the hardness of the steel surface, its wear resistance, corrosion resistance, acid resistance and other properties. Chemical-thermal treatment has found wide application in mechanical engineering, as it is one of the most effective methods of strengthening steel parts to increase their durability. Chemical-thermal treatment can be applied to parts of various sizes and shapes and a treated layer of the same thickness can be obtained. During chemical-thermal treatment, by changing the chemical composition of the surface layer, a large difference in the properties of the surface and core of the part is achieved. The disadvantage of chemical-thermal treatment processes is their low productivity. Chemical-thermal treatment is based on the diffusion of atoms of various chemical elements into the crystal lattice of iron when heated in a medium containing these elements. Chemical-thermal treatment consists of three processes: dissociation - obtaining a saturating element in an active atomic state: 2NH3↔2N+3H2, CH4↔C+2H2, etc.; absorption - absorption of active atoms of a saturating element by the surface of the metal; diffusion - movement of atoms of a saturating element from the surface into the depth of the metal.
It is necessary that the rates of all three processes be necessarily coordinated, and absorption and diffusion require that the saturating element interact with the base metal, forming either solid solutions or chemical compounds. Chemical-thermal treatment is impossible if the base metal and the saturating element form mechanical mixtures. The penetration depth of the diffusing element depends on the temperature and duration of saturation, as well as on the composition of the steel, mainly the presence of alloying elements. The most common types of chemical-thermal treatment are carburization (saturation of the surface layer with carbon), cyanidation (carbon and nitrogen), boriding (boron), aluminizing (aluminum), etc.
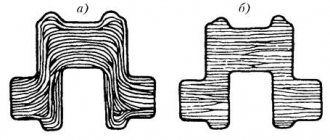
Cementation is a chemical-thermal treatment process consisting of diffusion saturation of the surface layer with carbon when heated in an appropriate environment. Cementation gives the surface layer high hardness and wear resistance, increases the endurance limit during bending and torsion. Parts that operate under friction conditions, under high pressures and cyclic loads are cemented: gears, piston pins, camshafts, etc. For cementation, low-carbon steels (0.1-0.3% C) are used, i.e. steel grades 10, 15 , 20, A12, A20, StZ, 15Kh, 25KhGM, etc. During cementation, the carbon content in the surface layer is brought to 1% (Fig. 45). The thickness (depth) of the cemented layer is 0.5-2.5 mm. For carbon steels, the depth of carburization is conventionally considered to be the distance from the surface of the part to half of the zone, the structure of which, along with pearlite, contains approximately the same amount of ferrite.
Rice. 45. Change in concentration (%) of carbon in the cemented surface layer
When carburizing, the part is heated without air access to 930-950°C in a carburizing medium (solid, liquid or gaseous), maintained at this temperature for several hours, and then slowly cooled. After this, it is subjected to normalization, hardening and tempering. The carburizing medium is solid carburizers (fine charcoal mixed with barium carbonate), liquid salt baths (a mixture of table salt, sodium carbonate, sodium cyanide and barium chloride) and gases containing carbon (natural, illuminating, etc.).
Cemented parts are subjected to hardening (820-850°C) and low tempering (150-170°C). After heat treatment, the structure of the surface layer is martensite or martensite with a small amount of carbides with a hardness of HRC 60-64. The core structure of parts made of carbon steels is ferrite, pearlite, and those of alloy steels are low-carbon martensite, troostite or sorbitol with a hardness of HRC 20-40, depending on the grade of steel and the size of the part.
Nitriding is a chemical-thermal treatment process that involves saturating the surface layer with nitrogen to give this layer high hardness, wear resistance or corrosion resistance. The hardness of the nitrided layer is higher than that of the cemented layer and is maintained up to high temperatures of 400-600°C, while the hardness of the cemented layer with a martensitic structure is maintained only up to 200-250°C. Alloy steels containing aluminum, chromium, titanium, for example 35ХМУА, 40Х, 18ХГТ, 40ХНМА, etc., are subjected to nitriding.
Before nitriding, the mechanical properties of parts are improved by subjecting them to hardening and high tempering. The thickness of the nitrided layer is 0.2-0.6 mm. The nitrided layer is well ground and polished. Nitriding is applied to car parts (gears, crankshafts), as well as dies, molds, etc. Nitriding leads to a slight increase in size. Therefore, after nitriding, the parts are subjected to final grinding (for example, the crankshaft journals are re-grinded) with a layer of 0.02-0.03 mm removed. Nitriding is usually carried out in ammonia at a temperature of 500-600ºC. Ammonia decomposes with the release of active nitrogen in the atomic state: 2NH3↔2N+6H. At these temperatures, in a hermetically sealed muffle inserted into the furnace, nitrogen penetrates into the surface layer of steel and enters into a chemical interaction with alloying elements, forming nitrides of chromium, molybdenum, tungsten, etc. Nitrides of alloying elements increase the hardness of steel to HRC 70. Conventional structural steels after nitriding they have lower hardness, and the hardness of carbon steels is quite low, since special nitrides are not formed in them. Therefore, carbon steels are subjected only to anti-corrosion nitriding.
The nitriding process is long: up to 24-60 hours at 500-520°C. The duration of the process can be reduced by two-stage nitriding. First, the temperature is maintained at 500-520°C, and the process is completed at 560-600°C. An increase in temperature, accelerating diffusion, reduces the time of formation of a layer of the required thickness without causing a decrease in surface hardness.
To reduce the duration of nitriding by 2-3 times, ion nitriding is used. The process is carried out in a rarefied nitrogen-containing atmosphere (NH3 or N2) when the workpiece is connected to the negative electrode - the cathode. The installation container serves as the anode. A glow discharge is excited between the part and the container, in which gas ions bombard the surface of the part. The duration of ion nitriding is from 1 to 24 hours. Nitriding in liquid media is carried out at 540-590°C in molten cyanide salts for 0.5-3 hours. With a total thickness of the nitrided layer of 0.15-0.5 mm, a thin layer is formed on the surface (7-15 microns) carbonitride layer with high wear resistance.
Nitrocarburization is a chemical-thermal treatment process that involves saturating the surface layer with both nitrogen and carbon in a gaseous environment. The basis of the gaseous environment is endothermic gas (endogas), consisting of nitrogen (40%), hydrogen (40%) and carbon monoxide (20%). When nitrocarburizing, parts are heated to 850-870ºC in an endogas environment with the addition of natural gas (5-15%) and ammonia (5%) and kept for 4-10 hours. The depth of the nitrocarburization layer is 0.2-0.8 mm. It depends on the process temperature and holding time. With increasing temperature, the nitrogen content in the layer decreases, and the carbon content increases up to a certain temperature, and then decreases slightly. After nitrocarburization, the parts are subjected to hardening and low tempering at 160-180°C to a hardness of HRC 58-64.
Nitrocementing is used for parts of complex shapes that are subject to wear (gears) and are prone to warping. Nitrocarburization has significant advantages over gas carburization due to a lower process temperature (by 70-90°C) and a smaller layer thickness, which ensures less deformation and warping of the part. Nitrocarburization is widely used in automobile and tractor production. Thus, at VAZ, up to 20% of parts undergoing chemical-thermal treatment are nitro-cemented.
Cyanidation is a chemical-thermal treatment process that involves saturating the surface layer with both nitrogen and carbon in molten salts containing sodium cyanide NaCN. To obtain a layer up to 0.3 mm thick, cyanidation is carried out at 820-860°C (low-temperature cyanidation) for 0.5-1.5 hours. Then the parts are hardened directly from the bath and subjected to low tempering (180-200°C). The hardness of the cyanidated layer after heat treatment is HRC 58-62. Parts made of medium-carbon steels and tools made of high-speed steel are subjected to low-temperature cyanidation. Low-temperature cyanidation is used to harden small parts.
The cyanidated layer has higher wear resistance compared to the cemented layer. To obtain a layer of greater thickness (0.5-2 mm), high-temperature cyanidation at 930-960°C is used. The duration of the process is 1.5-6 hours. After cyanidation, the parts are cooled in air, and then, to refine the grains, they are hardened and subjected to low tempering. High-temperature cyanidation is used for parts made of medium and low carbon, as well as alloy steels. Cyanide plating processes, in comparison with carburization, are more productive, provide less deformation and warping of parts of complex shapes and greater resistance to wear and corrosion. The disadvantage of cyanidation is the high cost and toxicity of cyanide salts.
Boridation is a chemical-thermal treatment process that involves saturating the surface layer with boron when heated in a boron-containing medium (borax, boron trichloride, etc.). Boriding is carried out at a temperature of 850-950°C for 2-6 hours. For boriding, low- and medium-carbon steels (20, 40, 45, 40Х, ZОХГС, etc.) can be used. The borated layer with a thickness of 0.1-0.2 mm has high hardness, wear resistance, especially in an abrasive environment, and corrosion resistance. Boriding is used to increase the wear resistance of parts of oil pumps, turbo drills, dies, molds, etc. Boriding increases the durability of parts by 2-10 times. Borated layers are highly brittle.
Diffusion metallization is a chemical-thermal treatment process in which the surface layer of steel is saturated with various metals (aluminum, chromium, zinc, etc.) and their complexes. When the steel surface is saturated with other metals, substitutional solid solutions are formed, so their diffusion is more difficult than the diffusion of carbon or nitrogen.
Diffusion saturation of the steel surface is carried out at temperatures of 700-1400°C by the following methods: 1. Solid diffusion metallization, in which the metallizer is a ferroalloy (ferrochrome, ferrosilicon, ferroaluminum, etc.) with the addition of ammonium chloride (NH4Cl). The metallizer, reacting with HCl or Cl2, forms a volatile chlorine compound with the metal (for example, AlCl3, CrCl2, etc.). As a result of contact with the metal surface, the volatile chlorine compound with the metal dissociates to form free atoms. 2. Liquid diffusion metallization, which is carried out by immersing the part in molten metal with a low melting point (zinc, aluminum). 3. Gas diffusion metallization, performed in a gas environment containing chlorides of various metals.
Aluminizing is the process of diffusion saturation of the surface layer of steel containing 0.1-0.2% C with aluminum. Aluminizing temperature is 700-1100°C. The thickness of the aluminized layer is 0.2-1 mm, and the concentration of aluminum in the surface layer is up to 30%. Aluminizing is used to increase the heat resistance of carbon steels. Thermocouple covers, parts of pouring ladles, valves and other parts operating at high temperatures are aluminized.
Chrome plating is the process of diffusion saturation of the surface layer with chromium. Chrome plating increases the scale resistance and wear resistance of parts in aggressive environments. Parts of steam turbines, pumps for pumping aggressive media, etc. are chrome plated.
Cold hardening and cold hardening
For some alloys, cold hardening is the only possible way to increase strength. Such alloys, for example, include corrosion-resistant alloys of chromium and nickel.
The study of a process such as cold hardening (hardening of metal) is one of the important and interesting problems of materials science. For example, as a result of cold hardening, the hardness of the surface layers of steel increases several times.
The terms cold hardening and cold hardening are often considered almost synonymous, meaning:
- the process of changing the structure of the material;
- increasing its hardness and strength as a result of these changes.
But in some literary sources these terms are distinguished: cold hardening is a process that can be either spontaneous or purposeful, while cold hardening is a deliberate process, the purpose of which is to strengthen the metal.
From this point of view, cold hardening can be a process both useful and harmful, and cold hardening is a process that can only be useful.
As the temperature increases, the cold hardening ability decreases noticeably. For example, cold hardening of aluminum is impossible at temperatures above 200 °C. This temperature (recrystallization temperature) will be different for different substances. For low-melting metals (including zinc, lead, tin), the recrystallization temperature can be negative.
Process description
Let us consider the essence of the phenomenon of cold hardening. As is known, almost all metals and their alloys (for example, aluminum or copper and their alloys) have an ordered crystalline structure. But it's not that simple. They consist of grains, within which the arrangement of atoms is ordered. But the grains themselves are located chaotically in relation to each other, that is, disordered.
Under mechanical load, dislocations (microscopic defects) appear in the structure of the substance. As the load increases, dislocations move and interact with each other.
Another structure is formed. It resists the deformation that remains after the load is removed (plastic deformation). The ability of the metal to resist deformation increases.
But it should be borne in mind that with cold hardening, the plastic properties of the material become worse. For example, the ductility of low-carbon steel decreases by 5-6 times. The resistance to plastic deformation also decreases when its sign changes (the so-called Bauschinger effect).
After hardening, the state of the substance is thermodynamically unstable. If ductility needs to be increased, hardening is removed by recrystallization annealing, heating the material above the recrystallization temperature. In this case, the material goes into a more stable state. The need to remove hardening arises, for example, in metallurgy during the production of wire or tape.
The dislocation density increases during work hardening, which leads to a decrease in bulk density. In this case, the metal grains are stretched in the direction of the forces that act on them. This grain orientation is called deformation texture. Due to texture, anisotropy of the mechanical properties of metals and alloys occurs.
The following conclusions can be drawn:
- after cold hardening or hardening, the hardness and strength of the material increases;
- The fragility of the material also increases.
In particular, cold hardening of steel is relevant for products in which it is necessary to prevent surface cracking and such phenomena as metal fatigue, which leads to the accumulation of internal stresses, the occurrence of cracks, and ultimately to the destruction of the material.
Types of hardening
Basically, there are two types of cold hardening:
- phase, when changes in the crystal lattice are caused by phase changes;
- deformation, when lattice changes are caused by external forces.
The formation of deformation hardening occurs when the surface being treated is exposed to balls or a stream of pellets.
Hardening equipment
Equipment for the process of cold hardening of aluminum and other metals and alloys is quite diverse. In industry, cold hardening is a fully automated process that is performed on electronically controlled devices.
In particular, when deformation hardening is formed, the number and feed rate of pellets is automatically adjusted.
Application
In industry, cold hardening is used to impart strength to products made of stainless steel, copper, aluminum and its alloys. This is very important for mechanical engineering, since various components and mechanisms often operate in unfavorable conditions and wear out over time.
HDTV steel hardening technology
The physical essence of the technology for hardening steels with high-frequency particles
The physics of the process of thermal strengthening of steels with high-frequency currents is based on the principle of induction induction of eddy currents, the so-called Foucault currents, in the surface layer of steel being strengthened, which, flowing through the conductor, are pushed away from the surface due to the skin effect and produce rapid heating of the surface layer of steel.
Then the heating stops and heat is removed deep into the metal. The rate of heat removal, that is, the cooling rate can exceed the rate of quenching for martensite
Most often, parts made of steel with a carbon content of 0.4-0.5% are subjected to surface hardening. These steels after hardening have a surface hardness of HRC55-60. With a lower carbon content, such hardness is no longer achieved, and with a higher carbon content, there is a danger of cracks appearing under conditions of sudden cooling with a water spray.
Advantages of HDTV steel hardening technology
Advantages of high-frequency hardening compared to volumetric furnace hardening and chemical-thermal treatment:
- high performance;
- high heating rate and the absence of exposure in the austenitic region makes it possible to obtain a higher dispersion of the structure of the hardened surface layer;
- increase in endurance limit: two to three times compared to volumetric (furnace) hardening;
- reduction of machine time for heat treatment;
- almost complete absence of scale on the hardened surface;
- reduction of warping during hardening;
- the possibility of complete mechanization and automation of the process (incorporating it into the processing production line, without interrupting the technological cycle).
Disadvantages of HDTV steel hardening technology
Like any hardening technology, induction hardening, in addition to its advantages, has certain limitations and disadvantages:
- depending on changes in the geometry of the part, it is necessary to change the geometry of the inductor;
- on parts with sharp corners, sharp geometric transitions, deep depressions, it is not possible to obtain a uniform thickness of the hardened layer;
- on some parts that have a complex geometric shape, manufacturing an inductor is difficult or practically impossible;
- HFC hardening causes slight deformation and warping of parts, especially with one-sided surface hardening of flat parts;
- In places where water hits the surface (during cooling with a spray), spider-shaped microcracks may appear. Microcracks under contact loading conditions are unacceptable, since they are the source of destruction.