Tin is a soft, silvery-white metal. It is so malleable and pliable that its sheets a thousandth of a millimeter thick can be rolled into a tube. This material is called tin paper. In D.I. Mendeleev’s periodic table of elements, this element corresponds to number 50, atomic weight 118.69 and the sign “Sn” (from the Latin stannum). There are 10 known stable isotopes. The metal is obtained mainly from the mineral cassiterite, which is tin dioxide.
The metal alloyed with lead is mainly used for soldering. It is also used as an anti-corrosion coating for food steel containers as it is non-toxic. Composites containing tin are used as fungicides, paints, toothpaste (SnF2) and ceramics.
Element history
This element was discovered in 1854 by Halus Pelegrin. However, its use began long before this date in the Middle East and Balkans around 2000 BC. During that era, bronze (an alloy of tin and copper) was discovered, which gave its name to the Bronze Age. They made weapons and tools from bronze, which were more effective than stone and bone.
In ancient times, the production of bronze led to the development of trade between different countries. There are also references to this metal in the Old Testament. Thus, in Mesopotamia they made bronze weapons, and in Ancient Rome they coated the inner surface of copper vessels with tin to increase their corrosion resistance.
General properties of tin
All the properties of this metal can be divided into two large groups: physical and chemical.
physical characteristics
It is a silvery, malleable metal that oxidizes easily at ambient temperatures, causing the color of tin to change to dark gray. If you bend a plate of this metal, you can hear a characteristic sound, the so-called “tin cry,” which arises due to friction between its constituent crystals. One of its pronounced characteristics is a sharp deterioration in mechanical properties under certain conditions, called “tin plague”: below a temperature of -18 ° C, the metal is destroyed, and it begins to look like a gray powder.
Pure tin has two allotropic modifications: gray and white. The gray modification has a cubic crystal structure, is a semiconductor, is very brittle, has low density and is stable at temperatures below 13.2 °C. The white allotropic modification has a tetragonal crystal structure, conducts electricity well and is stable at temperatures above 13.2 °C.
Metal melts at a relatively low temperature of 232 °C (for comparison: iron melts at 1535 °C). In this case, it is necessary to understand, when answering the question at what temperature tin melts, that it is its white allotropic modification that melts. Despite the low melting point, the metal boils at a relatively high temperature of 2602 °C (iron boils at 2750 °C).
Chemical properties
The most important mineral is cassiterite, SnO2. However, ore deposits with a high percentage of this mineral are currently unknown. Most of the world's cassiterite is mined from low-quality sediment deposits. It is from this mineral that tin is obtained on an industrial scale. To do this, cassiterite is crushed to obtain its concentrate, and then it is smelted along with coke, quartz and lime in a blast furnace. After this, the castings in the form of blocks undergo final cleaning to remove impurities of bismuth, copper and iron.
The chemical element tin reacts well with both strong acids and strong bases, but is relatively inert in neutral solutions. It is subject to corrosion in the presence of oxidizing environments; in the absence of oxygen, the metal practically does not corrode. During oxidation, a dense oxide film is formed on the surface of the metal, which protects the rest of it from further oxidation.
If an acidic environment is formed when salts are dissolved in water, then tin reacts in the presence of oxidizing agents or air. These salts include chlorides, for example, aluminum and iron. Most non-aqueous liquids, such as oils and alcohols, have little or no reaction with tin. Tin itself and its simple inorganic salts are not toxic, however, some organic composites are toxic.
Tin(II) oxide, SnO is a black-blue crystal that dissolves in acids and bases. It is used to produce salts in electroforming and glass production. Tin(IV) oxide, SnO2 is a white dust that is insoluble in acids. It is used as an indispensable component for coloring pink, yellow and brown ceramics, as well as in dielectrics and refractory alloys. It is an important agent in polishing marble and other decorative stones.
Tin(II) chloride, SnCl2 is the main ingredient in stannous acid for soldering. Tin(IV) chloride, SnCl4 is used as a chemical ingredient to add weight to silk fabric, as well as to stabilize some perfumes and color stabilize soaps, and SnF2, which is white in color and soluble in water, is used as an additive in toothpastes.
Organic chemical compounds based on this element are those in which at least one tin-hydrogen bond, Sn-H, is present, and in which the metal exhibits an oxidation state of +4. Organic compounds that have found their application in industry have the following chemical formulas:
- R4Sn;
- R3SnX;
- R2SnX2;
- RSnX3.
Here R is an organic group, for example, methyl, ethyl, butyl and others, and X is an inorganic element, for example, chlorine, oxygen, flor and others.
Melting copper at home
At home, copper alloys can be melted in several ways. When using any of the methods, you will need the following materials:
- crucible - a vessel made of hardened copper or other refractory metal;
- charcoal will be needed as a flux;
- metal hook;
- the shape of the future product.
The easiest option for melting is a muffle furnace. Pieces of material are dropped into the container. After setting the melting temperature, the process can be observed through a special window. The installed door allows you to remove the oxide film formed during the process; for this you need a pre-prepared metal hook.
The second way to melt at home is to use a torch or cutter. Propane - oxygen flame is perfect for working with zinc or tin. Pieces of materials for the future alloy are placed in a crucible and heated by the master with arbitrary movements. The maximum melting point of copper can be achieved when exposed to a blue flame.
Melting copper at home involves working at elevated temperatures. Compliance with safety regulations is a priority. Before any procedure, you should wear protective fireproof gloves and thick clothing that completely covers the body.
Tin-based alloys
Tin-based alloys are also known as white metals and typically contain copper, antimony and lead. Alloys have different mechanical properties depending on their composition.
Tin-lead alloys have found commercial use in a wide range of compositions. Thus, 61.9% tin and 38.1% lead correspond to a eutectic composition, the solidification degree of which is 183 °C. Alloys with a different ratio of these metals melt and crystallize over a wide temperature range, when there is an equilibrium between the solid and liquid phases. With such crystallization, solid segregations begin to form in the melt, which lead to the formation of various structures. An alloy of eutectic composition, since it has the lowest melting point, is used as a fuse against overheating of electronic components.
There are also alloys in which, in addition to the indicated metals, there is a small amount of antimony (up to 2.5%). The main problem with alloys based on tin and lead is their negative impact on the environment, so recently their substitutes have been developed that do not use lead, for example, alloys with silver and copper.
Alloys of tin, lead and antimony are used for decorative ornaments, and some alloys of tin, copper and antimony are used as a lubricant to reduce friction in bearings due to their antifriction properties. In addition to the above alloys, tin is used in bronze alloys and in alloys with titanium and zirconium.
Determination of specific heat of fusion
The specific heat of fusion (designated by the Greek letter “lambda” - λ) is a physical quantity equal to the amount of heat (in joules) that must be transferred to a solid body weighing 1 kg in order to completely transform it into the liquid phase. The formula for the specific heat of fusion looks like this:
$$ λ ={Q over m}$$
Where:
m is the mass of the melting substance;
Q is the amount of heat transferred to the substance during melting.
Values for different substances are determined experimentally.
Knowing λ, we can calculate the amount of heat that must be imparted to a body of mass m for its complete melting:
$$Q={λ*m}$$
Using the element and its connections
All areas of human production in which this element is directly or indirectly used are listed below:
- Protection against corrosion and mechanical impact of steels and other metals, for example, in the production of cans;
- Reducing the fragility of glass, as well as in the production of mirrors;
- In chased patterns on various dishes;
- Use in fungicides, paints, toothpastes and various pigments.
- When producing various alloys, for example bronze.
- For low temperature or soft soldering;
- Contains lead in the production of metal sheets for musical instruments;
- In the production of labels for various products;
- In alloys that protect electrical devices and electronic microcircuits from overheating;
- In the ceramic industry for the production of enamels as a matting agent.
- In capsules for sealing wine bottles. The production of such capsules expanded after the ban on the use of lead in the food industry.
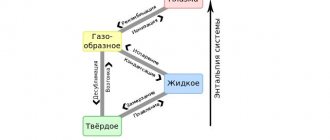
Melting and crystallization
Details Category: Molecular-kinetic theory Published 06.11.2014 13:52
The same substance under certain conditions can be in different states of aggregation - solid, liquid or gaseous. When transitioning from one state to another, the composition of the molecules of this substance does not change. Only their location, the nature of thermal movement and the forces of intermolecular influence change.
A substance passes from a solid state to a liquid state, and from a liquid state to a gaseous state. This transition is called a phase transition
.
Effects of exposure to tin compounds
The activity of compounds with this element, one way or another, affects both the human body and the environment.
On human health
As already mentioned, the most dangerous to human health are organic chemical compounds of tin. These substances are widely used in industry, for example, in the production of paints, plastics and agricultural pesticides. In addition, the production volumes of organic compounds with this metal are constantly growing, despite the fact that the consequences of poisoning with them are known.
The effects of these substances on humans are varied, it all depends on the type of compound and on the individual characteristics of the body. The danger of a compound correlates with the length of the bond between the metal and the hydrogen; the longer the bond, the less dangerous the compound. In this regard, the most dangerous organic substance is considered to be a tin compound with three ethyl groups, the hydrogen bonds of which are relatively short.
These substances can enter the human body through food, airborne droplets, or simply touching them. The following effects of organic tin compounds on the human body are known:
- If you are in a room containing vapors of this metal, severe irritation of the upper respiratory tract, skin and eyes;
- Headaches, stomach pain and lack of appetite;
- Nausea and vomiting;
- Problems with urination;
- Heavy sweating and shortness of breath.
The following effects can lead to more serious consequences:
- Depression;
- Liver problems;
- Immune system dysfunction;
- Damage to cell chromosomes and lack of red cells in the blood;
- Brain damage (sleep disturbances, headaches, memory loss, irritability).
On the environment
Both tin atoms and the metal itself in a pure state are not toxic to any organism on earth, in turn, almost all compounds with this element of an organic nature are harmful. These compounds can remain in the environment for long periods of time. They are quite stable and practically do not decompose under the influence of microorganisms, due to their strong hydrogen bonds. No matter how small the concentrations of this metal compounds in soil and water are, in view of the above, they are constantly growing.
Organotin compounds are known to cause great harm to aquatic ecosystems as they are toxic to fungi, algae and phytoplankton. Phytoplankton is an important part of the aquatic ecosystem, since it produces oxygen for all other living organisms in this system, and is also an important part in the food chain. The toxicity of tin compounds varies among living things, for example, tributyl tin is poisonous to fish and fungi, while the most toxic compound to phytoplankton is triphenol tin.
It is also known that organic compounds of this element have a negative effect on the growth and reproductive function of animals and disrupt the functioning of enzymes. Such compounds accumulate mainly in the upper layers of soil and water.
Melting point table
To find out what temperature is needed to melt metals, a table of increasing temperature indicators will help.
| Element or connection | Required temperature conditions |
| Lithium | +18°С |
| Potassium | +63.6°С |
| Indium | +156.6°С |
| Tin | +232°С |
| Thallium | +304°С |
| Cadmium | +321°С |
| Lead | +327°С |
| Zinc | +420°С |
Melting table for medium-melting metals and alloys.
| Element or alloy | Temperature |
| Magnesium | +650°С |
| Aluminum | +660°С |
| Barium | +727°С |
| Silver | +960°С |
| Gold | +1063°С |
| Manganese | +1246°С |
| Copper | +1083°С |
| Nickel | +1455°С |
| Cobalt | +1495°С |
| Iron | +1539°С |
| Durali | +650°С |
| Brass | +950…1050°С |
| Cast iron | +1100…1300°С |
| Carbon steels | +1300…1500°С |
| Nichrome | +1400°С |
Melting table for refractory metals and alloys.
| Item name | Temperature |
| Titanium | +1680°С |
| Platinum | +1769.3°С |
| Chromium | +1907°С |
| Zirconium | +1855°С |
| Vanadium | +1910°С |
| Iridium | +2447°С |
| Molybdenum | +2623°С |
| Tantalum | +3017°С |
| Tungsten | +3420°С |