Metals and many other materials can be in a solid or liquid state. When exposed to a certain temperature, the crystal lattice of the metal is transformed, which leads to an increase in ductility and a decrease in hardness . Due to this shape, various alloys and cast products are obtained. However, a low melting point is not always a positive quality of a material. In some cases, the manufactured product must withstand heat during operation. Let's consider what the melting temperature of a metal can be in degrees and what this indicator depends on.
Melting point table
To find out what temperature is needed to melt metals, a table of increasing temperature indicators will help.
| Element or connection | Required temperature conditions |
| Lithium | +18°С |
| Potassium | +63.6°С |
| Indium | +156.6°С |
| Tin | +232°С |
| Thallium | +304°С |
| Cadmium | +321°С |
| Lead | +327°С |
| Zinc | +420°С |
Melting table for medium-melting metals and alloys.
| Element or alloy | Temperature |
| Magnesium | +650°С |
| Aluminum | +660°С |
| Barium | +727°С |
| Silver | +960°С |
| Gold | +1063°С |
| Manganese | +1246°С |
| Copper | +1083°С |
| Nickel | +1455°С |
| Cobalt | +1495°С |
| Iron | +1539°С |
| Durali | +650°С |
| Brass | +950…1050°С |
| Cast iron | +1100…1300°С |
| Carbon steels | +1300…1500°С |
| Nichrome | +1400°С |
Melting table for refractory metals and alloys.
| Item name | Temperature |
| Titanium | +1680°С |
| Platinum | +1769.3°С |
| Chromium | +1907°С |
| Zirconium | +1855°С |
| Vanadium | +1910°С |
| Iridium | +2447°С |
| Molybdenum | +2623°С |
| Tantalum | +3017°С |
| Tungsten | +3420°С |
What is melting point
Each metal has unique properties, and this list includes its melting point. When melting, the metal changes from one state to another, namely, from solid to liquid. To fuse a metal, you need to bring heat closer to it and heat it to the required temperature - this process is called the melting point. At the moment when the temperature reaches the desired level, it can still remain in a solid state. If you continue the impact, the metal or alloy will begin to melt.
Interesting: Description and types of welded joints
Melting and boiling are not the same thing. The point at which a substance transitions from solid to liquid is often referred to as the melting point of the metal. In the molten state, the molecules do not have a specific arrangement, but attraction holds them close together; in liquid form, the crystalline body leaves volume, but the shape is lost.
During boiling, the volume is lost, the molecules interact very weakly with each other, move chaotically in different directions, and separate from the surface. Boiling point is the process by which the pressure of metal vapor is equal to the pressure of the external environment.
In order to simplify the difference between critical heating points, we have prepared a simple table for you:
| Property | Melting temperature | Boiling temperature |
| Physical state | The alloy transforms into a melt, the crystalline structure is destroyed, and granularity passes through | Transforms into a gas state, some molecules can fly away outside the melt |
| Phase transition | Equilibrium between solid and liquid | Pressure equilibrium between metal vapor and air |
| Influence of external pressure | No changes | There are changes, the temperature decreases with discharge |
At what temperature does it melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
Different substances have different melting points. Theoretically, metals are divided into:
- Low-melting - temperatures up to 600 degrees Celsius are sufficient to obtain a liquid substance.
- Medium melting - requires a temperature of 600 to 1600 ⁰C.
- Refractory are metals that require temperatures above 1600 ⁰C to melt.
Melting iron
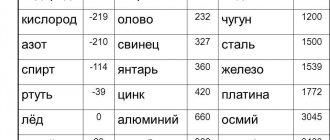
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Interesting: How to cook cast iron
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray - the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White - the temperature reaches 1350 degrees. It is poured into molds at 1450.
Important! The melting rates of a metal such as cast iron are 400 degrees lower compared to steel. This significantly reduces energy costs during processing.
Melting steel
Melting steel at a temperature of 1400 °C
Steel is an alloy of iron with an admixture of carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Reference! Steel melts at 1400 °C.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Density:
Depending on their density, metals are divided into light (density from 0.53 to 5 g/cm³) and heavy (from 5 to 22.6 g/cm³).
The lightest metal is lithium (density 0.53 g/cm³). It is currently impossible to name the heaviest metal, since the densities of osmium and iridium - the two heaviest metals - are almost equal (about 22.6 g / cm³ - exactly twice the density of lead), and it is extremely difficult to calculate their exact density: for To do this, the metals must be completely purified, because any impurities reduce their density.
What does melting temperature depend on?
For different substances, the temperature at which the structure is completely reconstructed to the liquid state is different. If we take into account metals and alloys, then it is worth noting the following points:
- Metals are not often found in their pure form. The temperature directly depends on its composition. As an example, we will indicate tin, to which other substances (for example, silver) can be added. Impurities make the material more or less resistant to heat.
- There are alloys that, due to their chemical composition, can transform into a liquid state at temperatures above one hundred and fifty degrees. There are also alloys that can “hold” when heated to three thousand degrees and above. Taking into account the fact that when the crystal lattice changes, the physical and mechanical qualities change, and operating conditions can be determined by the heating temperature. It is worth noting that the melting point of a metal is an important property of a substance. An example of this is aviation equipment.
Interesting: Welds - defects and their elimination
Heat treatment, in most cases, almost does not change the resistance to heat. The only sure way to increase resistance to heat is to make changes to the chemical composition; this is why steel is alloyed.
Solid and liquid state of metal
Many people are familiar with metals and alloys by their solid state. They are found in almost all areas of activity. Only in metallurgy and in production shops is metal found in a liquid state. This is due to the fact that in order to transform the crystal lattice it is necessary to heat the raw material to record temperatures.
The solid state is characterized by the following qualities:
- The structure holds its shape. Steel is known for being able to withstand heavy loads over long periods.
- Each material has its own characteristics of strength, hardness, and toughness .
- Constant chemical composition. The surface of steel or other alloys may react to chemicals, oxidize or become corroded, but the chemical composition remains unchanged.
- Possibility of cutting. With an increase in plasticity, chips are not formed at the time of machining, which significantly complicates the process.
In a liquid or viscous state, the metal acquires completely different properties:
- High plasticity allows for mold casting, forging, or other processing associated with plastic deformation of workpieces.
- It is possible to change the chemical composition by adding alloying elements. Due to the movable crystal lattice, it is possible to saturate the steel structure with chromium, nickel, titanium and many other substances.
- Heat treatment is also carried out at a temperature that leads to a restructuring of the crystal lattice. However, when hardened, the metal retains its shape , that is, the structure remains solid.
There are alloys that can be heated to a liquid state at home. An example is tin, used in the manufacture of solder. The melting point of tin is within 250 degrees Celsius. This heating rate can be achieved using a conventional soldering iron .
Which metal has the highest melting point
Tungsten is the most refractory metal, 3422 °C (6170 °F).
A hard, refractory, fairly heavy material of light gray color that has a metallic sheen. It is difficult to machine. At room temperature it is quite fragile and breaks. The fragility of the metal is associated with contamination with carbon and oxygen impurities.
Note! Technically, pure metal at temperatures above four hundred degrees Celsius becomes very ductile. Demonstrates chemical inertness and is reluctant to react with other elements. It occurs in nature in the form of such complex minerals as: hübnerite, scheelite, ferberite and wolframite.
Tungsten can be obtained from its ore, through complex chemical processing, as a powder. Using pressing and sintering, it is used to create parts of regular shape and bars.
Tungsten is an extremely resistant element to any temperature influences. For this reason, tungsten could not be softened for more than a hundred years. There was no furnace that could heat up to several thousand degrees Celsius. Scientists have been able to prove that this is the most refractory metal. Although there is an opinion that seaborgium, according to some theoretical data, has greater refractoriness, this is only an assumption, since it is a radioactive element and has a short lifespan.
Types of metal alloys
The types of metal alloys vary based on their melting point, so the alloy variants are as follows:
- Low-melting (tin, zinc, lead, bismuth) with a melting point of no more than 600 °C.
- Medium melting (aluminium, magnesium, nickel, iron) with a temperature of 600 – 1,600 °C.
- Refractory (molybdenum, tungsten, titanium) with a temperature of more than 1,600 °C.
Next, we’ll tell you a little about the types of steels, wood alloy and solders.
Features of Carbon Steel
This material contains an admixture of carbon, approximately 2.13%. Moreover, it is devoid of alloying additives, but contains impurities of silicon, manganese and magnesium.
Features of Alloy Steel
In addition to the carbon and iron content, additional elements are added to it to improve its properties.
Features of stainless steel
Stainless steel differs from carbon steel due to the content of the element chromium in its composition, due to the properties of which it is not susceptible to oxidation, and, therefore, to rust.
Features of tool steel
It also has a carbon composition (0.8 - 0.9%). Demonstrates hardness, strength, and is easy to process. Used in the manufacture of instruments, such as medical ones.
Wood's alloy
It is a material used in soldering parts for radio receivers, as well as in electroplating, when working in laboratory conditions with pesticides.
Soldering alloys
Another name for them is solders. Materials for solders are different. It all depends on what is included in the materials that need to be combined. For example, aluminum requires one alloy of solder, but copper is completely different.