Chemistry tutorial › Inorganic chemistry
It is known that all simple substances can be divided into simple substances - metals and simple substances - non-metals.
METALS, as defined by M.V. Lomonosov, are “light bodies that can be forged.” These are usually malleable, shiny materials with high thermal and electrical conductivity. These physical and many chemical properties of metals are related to the ability of their atoms to GIVE UP electrons.
NON-METALS, on the contrary, are able to ADD electrons in chemical processes. Most nonmetals exhibit the opposite properties of metals: they do not shine, do not conduct electricity, and are not forged. Being opposite in properties, metals and non-metals easily react with each other.
This part of the Self-Teacher is devoted to a brief overview of the properties of metals and non-metals. When describing the properties of elements, it is advisable to adhere to the following logical scheme:
1. First, describe the structure of the atom (indicate the distribution of valence electrons), draw a conclusion about whether this element belongs to metals or non-metals, determine its valence states (oxidation states) - see lesson 3;
2. Then describe the properties of a simple substance by composing reaction equations
- with oxygen;
- with hydrogen;
- with metals (for non-metals) or with non-metals (for metals);
- with water;
- with acids or alkalis (where possible);
- with salt solutions;
3. Then you need to describe the properties of the most important compounds (hydrogen compounds, oxides, hydroxides, salts). In this case, you must first determine the nature (acidic or basic) of a given compound, and then, remembering the properties of compounds of this class, draw up the necessary reaction equations;
4. And finally, it is necessary to describe the qualitative reactions to cations (anions) containing this element, methods for obtaining a simple substance and the most important compounds of this chemical element, and indicate the practical application of the studied substances of this element.
So, if you determine that an oxide is acidic, then it will react with water, basic oxides, bases (see lesson 2.1) and it will correspond to an acidic hydroxide (acid). When describing the properties of this acid, it is also useful to look at the corresponding section: lesson 2.2.
Internal structure and physical properties of metals
Metals are simple substances whose atoms can only give up electrons. This feature of metals is due to the fact that at the outer level of these atoms there are few electrons (most often from 1 to 3) or the outer electrons are located far from the nucleus. The fewer electrons at the outer level of the atom and the further they are located from the nucleus, the more active the metal (the more pronounced its metallic properties).
Task 8.1. Which metal is more active:
Name the chemical elements A, B, C, D.
Metals and non-metals in Mendeleev's Periodic Table of Chemical Elements (PSM) are separated by a line drawn from boron to astatine. Above this line in the main subgroups are non-metals (see lesson 3). The remaining chemical elements are metals.
Task 8.2. Which of the following elements are metals: silicon, lead, antimony, arsenic, selenium, chromium, polonium?
Question. How can we explain the fact that silicon is a non-metal, and lead is a metal, although they have the same number of outer electrons?
An essential feature of metal atoms is their large radius and the presence of valence electrons weakly bound to the nucleus. For such atoms, the ionization energy* is small.
* IONIZATION ENERGY is equal to the work spent on removing one external electron from an atom (to ionize an atom) that is in the ground energy state.
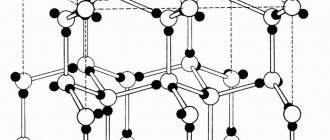
Some of the valence electrons of metals, breaking away from atoms, become “free”. “Free” electrons easily move between atoms and metal ions in the crystal, forming an “electron gas” (Fig. 28).
At a subsequent moment in time, any of the “free” electrons can be attracted by any cation, and any metal atom can give up an electron and turn into an ion (these processes are shown in Fig. 28 by dotted lines).
Thus, the internal structure of a metal is similar to a layer cake, where positively charged “layers” of metal atoms and ions alternate with electronic “layers” and are attracted to them. The best model of the internal structure of a metal is a stack of glass plates moistened with water: it is very difficult to tear one plate from another (strong metals), and it is very easy to move one plate relative to another (ductile metals) (Fig. 29).
Task 8.3. Make such a “model” of the metal and verify these properties.
A chemical bond carried out by “free” electrons is called a metallic bond .
“Free” electrons also provide such physical properties of metals as electrical and thermal conductivity, plasticity (malleability), as well as metallic luster.
Task 8.4. Find metal objects around the house.
By completing this task, you can easily find metal utensils in the kitchen: pots, pans, forks, spoons. Machine tools, airplanes, cars, diesel locomotives, and tools are made from metals and their alloys. Modern civilization is impossible without metals, since electrical wires are also made of metals - Cu and Al. Only metals are suitable for making antennas for radio and television receivers; the best mirrors are made from metals. In this case, not pure metals are often used, but their mixtures (solid solutions) - ALLOYS.
Defects in crystals
Crystals always have structural defects caused by a violation of the arrangement of atoms in the crystal lattice. Defects in the crystal structure are divided into point , linear and surface .
The reason for the formation of defects is vacancies (the place where an atom with higher energy was located and moved from one place to another). After some time, one of the atoms of the neighboring layer moves to this vacancy site, etc. Thus, the vacancy moves deeper into the crystal. With increasing temperature, the number of vacancies increases, and they move more often from one site to another. Point defects also include an atom embedded in an interstitial site of a crystal lattice, and a substituted atom, when the place of an atom of one metal is replaced in the crystal lattice by another, foreign atom. Point defects cause local distortion of the crystal lattice. Linear defects are another important type of imperfection of a crystal lattice, when, as a result of a shift of one interatomic distance of one part of the lattice relative to another along a certain plane, the number of rows of atoms in the upper part of the lattice is one more than in the lower part. In this case, an extra atomic plane ( extraplane ) appeared in the upper part of the lattice. The edge of the extraplane, perpendicular to the shear direction, is called edge (linear) dislocation , the length of which can reach many thousands of interatomic distances. The crystal lattice in the dislocation zone is elastically distorted, since the atoms in this zone are displaced relative to their equilibrium state.
Surface defects represent interfaces between individual crystals. At the interface, the atoms of the crystal are not as ordered as in its bulk. In addition, dislocations and vacancies accumulate along the interfaces, and impurities are concentrated, which further disrupts the arrangement of atoms. In this case, the crystals themselves are misoriented, i.e. can be rotated relative to each other by tens of degrees.
Defects in crystals significantly affect the properties of metals. To obtain high-quality metals and alloys, it is necessary to reduce all defects to a minimum. This can be achieved by resorting to special processing methods, such as heat treatment methods.
Alloys
Metals easily form alloys - materials that have metallic properties and consist of two or more chemical elements (simple substances), at least one of which is a metal. Many metal alloys have a single metal as the base with small additions of other components. In principle, it is difficult to draw a clear boundary between metals and alloys, since even the purest metals contain “trace” impurities of other chemical elements.
All the items listed above - machines, airplanes, cars, frying pans, forks, spoons, jewelry - are made from alloys. Impurity metals (alloying components) very often change the properties of the base metal for the better, from a human point of view. For example, both iron and aluminum are fairly soft metals. But when combined with each other or with other components, they turn into steel, duralumin and other durable structural materials. Let's look at the properties of the most common alloys.
Steel is an alloy of iron and carbon, containing up to 2% of the latter. Alloy steels also contain other chemical elements - chromium, vanadium, nickel. There are far more steels produced than any other metals and alloys, and it is difficult to list all of their possible uses. Low-carbon steel (less than 0.25% carbon) is consumed in large quantities as a structural material, and steel with a higher carbon content (more than 0.55%) is used to make cutting tools: razor blades, drills, etc.
Iron forms the basis of cast iron . Cast iron is an alloy of iron with 2–4% carbon. Silicon is also an important component of cast iron. A wide variety of very useful products can be cast from cast iron, such as manhole covers, pipeline fittings, engine cylinder blocks, etc.
Bronze is an alloy of copper, usually with tin as the main alloying component, and also with aluminum, silicon, beryllium, lead and other elements, excluding zinc. Tin bronzes were known and widely used in ancient times. Most antique bronzes contain 75-90% copper and 25-10% tin, which makes them look similar to gold, but they are more refractory. This is a very durable alloy. Weapons were made from it until they learned how to produce iron alloys. An entire era in human history is associated with the use of bronze: the Bronze Age.
Brass is an alloy of copper with Zn, Al, Mg. These are non-ferrous alloys with a low melting point and are easy to process: cut, weld and solder.
Cupronickel is an alloy of copper and nickel, sometimes with the addition of iron and manganese. In terms of external characteristics, cupronickel is similar to silver, but has greater mechanical strength. The alloy is widely used for making tableware and inexpensive jewelry. Most modern silver-colored coins are made from cupronickel (usually 75% copper and 25% nickel with minor additions of manganese).
Duralumin , or duralumin, is an aluminum-based alloy with the addition of alloying elements - copper, manganese, magnesium and iron. It is characterized by its steel strength and resistance to possible overloads. It is the main structural material in aviation and astronautics.
- 1. Structure of metals
- 2. Crystallization and structure of metals and alloys
- 3. Diffusion and non-diffusion transformations
- 4. Classification of alloys. Iron and its alloys
- 5. State diagrams of alloys
LECTURE No. 5. Alloys
1. Structure of metals
Metals and their alloys
– the main material in mechanical engineering. They have many valuable properties, mainly due to their internal structure. A soft and ductile metal or alloy can be made hard and brittle, and vice versa. In order to consciously change the properties of metals, it is necessary to know the basics of their crystal structure. As is known, all bodies consist of a large number of atoms, which are held together by cohesive forces, oscillating at high frequencies near equilibrium points. Since the atoms of different metals are different, each metal has its own specific properties. These properties depend on the arrangement of atoms among themselves, the nature of their bonds, and the distance between them. If you change the distance between atoms or the order of their arrangement, the properties of the metal will also change. In amorphous bodies - resin, glass, rosin, etc. - the atoms are arranged randomly. In metals they are in a certain geometric order, forming crystals, which is why metals are crystalline solids. Metals differ not only in the order of arrangement of atoms, but also in their crystal lattice, which is an imaginary spatial grid consisting of elementary cells, at the nodes of which atoms are located.
The following crystal lattices of metals with close packing of atoms are distinguished: body-centered cubic, face-centered cubic, and hexagonal.
In a cell of a cubic body-centered lattice, the atoms are located at the vertices and center of the cube. Such a cell contains nine atoms (chromium, tungsten, vanadium, molybdenum, lithium, and at certain temperatures, iron and other metals).
In a cell of a cubic face-centered lattice, atoms are located at the vertices of the cube and at the intersection of the diagonals of each plane. Such a cell has 14 atoms (lead, nickel, copper, gold, silver, plate, iron at certain temperatures and other metals).
In a cell of a hexagonal crystal lattice, atoms are located at the vertices and in the center of the hexagonal bases of the prism, and three atoms are located in its middle plane, while such a cell contains 17 atoms (magnesium, zinc, cadmium, osmium, beryllium and other metals).
Under certain conditions, some metals - iron, titanium, zirconium, strontium, cobalt, calcium and others can be rearranged from one type of crystal lattice to another, for example, from a body-centered cubic to a face-centered and even hexagonal one. The unit cell represents only one element, or one cell, of the crystal lattice.
The entire crystal lattice in a real metal consists of a large number of repeated unit cells. The distance between the atoms of a crystal lattice cell or between parallel atomic planes forming a unit cell is of great importance. The greater this distance, the less durable the metal. The distance between them is measured in angstroms - 1 A = 10 -8 cm or in nanometers - 1 A = 0.1 nm.
It is known from practice that iron is stronger than copper, and copper is stronger than aluminum.
2. Crystallization and structure of metals and alloys
The order of arrangement of atoms - the type of crystal lattice - a natural property of the metal, the shape of the crystals and their sizes depend on the process of transition of the metal from a liquid to a solid state. The process of crystal formation when metals solidify is called crystallization.
When metals crystallize, heat is released, and when metals transition from solid to liquid, heat is absorbed. Observations of the process of temperature decrease using temperature-measuring devices
during the transition of a metal from a liquid to a solid state, they made it possible to establish a certain pattern. At first, the temperature decreases evenly. During the initial period of crystal formation, due to the release of latent heat during the formation of the crystal lattice, the temperature drop stops, and it remains unchanged until the metal is completely solidified. After all the metal has solidified, the temperature begins to drop again. The temperature corresponding to the horizontal area is called critical.
The crystallization of metals is similar to the crystallization of salts, and this process consists of two elementary processes occurring simultaneously. The first is the formation of crystallization centers, or crystal nuclei, the second is the growth of crystals from these centers.
First stage
– appearance of metal crystal nuclei.
The second stage
- as the metal cools, more and more new atoms of the liquid metal join the nuclei, which are grouped in a certain order next to each other, forming elementary cells of the crystal lattice. This process continues until crystallization is complete. Moreover, the crystals of the solidified metal have an irregular and very diverse shape, which is explained by the crystallization conditions.
During the crystallization process, the number of crystals increases - over 1000 crystals can form in 1 mm3. Crystals that have an irregular external shape are called crystallites.
or grains.
Pure metals are relatively rarely used in mechanical engineering and other sectors of the economic complex. More widely used are alloys consisting of two or more elements (two metals, such as copper and zinc, or a metal and a non-metal, such as iron and carbon). The elements included in the alloy are called components.
Depending on the arrangement of atoms in the crystal lattice, substitutional solid solutions and interstitial solid solutions are distinguished. In a substitutional solid solution, the atoms of the soluble component are replaced by solvent atoms, and in an interstitial solid solution, the solvent atoms are located between the atoms of the soluble component in the weakest places of the elements of the crystal lattice.
Alloys that are solid solutions have valuable properties. They are harder and stronger than the components included in it.
During crystallization, the components of some alloys can enter into a chemical bond, forming a chemical compound. Chemical compounds have very high hardness and good electrical resistance.
3. Diffusion and non-diffusion transformations
Under diffusion
understand the movement of atoms in a crystalline body over distances exceeding the average interatomic distances of a given metal.
If the movements of atoms are not associated with changes in concentration in individual volumes, then such a process is called self-diffusion.
Diffusion accompanied by a change in concentration is called
heterodiffusion.
In cases where heterodiffusion is accompanied by the formation of new phases, which most often occurs during chemical processing, it is called
reactive diffusion.
The diffusion process is based on an atomic mechanism in which each atom performs more or less random walks. Diffusion transformations in metals occur during various chemical and thermal treatments - chrome plating, carburization, aluting
(aluminizing), etc.
Chrome plating
provides increased heat resistance of steel up to 800 °C, high corrosion resistance in environments such as fresh and sea water, acetic and phosphoric acids, and erosion resistance at low and high temperatures.
Chromium plating of steels containing more than 0.3–0.4% carbon also increases hardness and wear resistance. When chrome plating, the diffusion layer consists of a solution of chromium in? – iron, and the chromium content on the surface is 25–50%.
In this process, when CrCl 2 is used, the following reaction occurs:
CrCl 2 + Fe > FeCl 2 + Cr.
During heat treatment of steel, diffuse-free,
or
allotropic,
transformations during the process of secondary crystallization. In particular, at a temperature of +775 °C in steel containing 0.6% carbon, allotropic transformations begin, i.e., the separation of ferrite from austenite (solid solution of carbon (up to 2.14%)) and other impurities in the bulk of iron.
Ferrite
– solid solution of a small amount of carbon (up to 0.04%) and other impurities in? – gland – a soft, plastic and insufficiently strong structural component. Since ferrite contains an insignificant amount of carbon, the remaining austenite will gradually become enriched in carbon as ferrite is released. When the carbon concentration in the remaining austenite reaches 0.8%, at a temperature of +727 °C steel containing 0.6% carbon will contain ferrite and austenite, and at temperatures below +727 °C - ferrite and pearlite, and the ferrite-pearlite structure will remain unchanged without significant changes even with further cooling of the steel down to room temperature. Similar transformations are characteristic of all hypoeutectoid steels (containing less than 0.8% carbon). The only difference will be in the temperatures at which ferrite begins to precipitate. Moreover, if the steel contains 0.8% carbon, its secondary crystallization will occur at a constant temperature (+727 °C) and will be accompanied by only one process - the formation of pearlite. This is explained by the fact that in this case the carbon content in the steel corresponds to the eutectoid composition - a mechanical mixture of crystals released from the liquid alloy simultaneously. This creates a fine-grained structure of the alloy.
4. Classification of alloys. Iron and its alloys
Steel and cast iron
– basic materials in mechanical engineering. They make up 95% of all alloys used in technology.
Steel
is an alloy of iron with carbon and other elements, containing up to 2.14% carbon.
Carbon
is the most important impurity in steel. The strength, hardness and ductility of steel depend on its content. In addition to iron and carbon, steel also contains silicon, manganese, sulfur and phosphorus. These impurities enter the steel during the smelting process and are its inevitable companions.
Cast iron
– iron-based alloy. The difference between cast iron and steel is its higher carbon content - more than 2.14%. The most widespread are cast irons containing 3–3.5% carbon. Cast iron contains the same impurities as steel, i.e. silicon, manganese, sulfur and phosphorus. Cast irons in which all the carbon is in a chemical combination with iron are called white (by the type of fracture), and cast irons in which all or most of the carbon is graphite are called gray. White cast iron always contains one more structural component - ledeburite. This is a eutectic, i.e. a uniform mechanical mixture of austenite and cementite grains obtained during the crystallization process, it contains 4.3% carbon. Ledeburite is formed at a temperature of +1147 °C.
Ferrite
– solid solution of a small amount of carbon (up to 0.04%) and other impurities in?
– gland. It's practically pure iron. Cementite
is a chemical compound of iron and carbon – iron carbide.
Perlite
– a uniform mechanical mixture in an alloy of ferrite and cementite. This mixture received this name because the thin section during etching has a pearlescent tint. Since pearlite is formed as a result of secondary crystallization processes, it is called a eutectoid. It is formed at a temperature of +727 °C. It contains 0.8% carbon.
Perlite comes in two varieties. If the cementite in it is located in the form of plates, it is called lamellar, but if the cementite is located in the form of grains, perlite is called granular. Under a microscope, cementite plates appear shiny because they have great hardness, are well polished, and are less corroded by acid etching than soft ferrite plates.
If iron-carbon alloys are heated to certain temperatures, an allotropic transformation will occur? -iron in ? -iron and a structural component is formed, which is called austenite.
Austenite
is a solid solution of carbon (up to 2.14%) and other impurities in? -iron. Carbon ability
dissolution in iron varies at different temperatures. At a temperature of +727 °C? —iron can dissolve no more than 0.8% carbon. At the same temperature, austenite decomposes to form pearlite. Austenite is a soft structural component. It is characterized by great plasticity and does not have magnetic properties.
When studying the structural components of iron-carbon alloys, it was found that at room temperature they always consist of two structural elements: soft plastic ferrite and hard cementite, which strengthens the alloy.
5. State diagrams of alloys
Alloys can be made by combining most metals with each other, as well as with non-metals. State diagrams of alloys provide a visual representation of the transformations occurring in alloys depending on their chemical composition and temperature.
When constructing phase diagrams of alloys, the chemical composition or concentration of the alloy as a percentage is indicated on the abscissa axis. To do this, a horizontal line of a certain length is divided into one hundred identical parts and each division is taken as 1% of one of the components of the alloy.
Rice. 5. State diagram of alloys of the lead-antimony (Pb-Sb) system
Point A
corresponds to pure lead, and point
B
corresponds to pure antimony. The ordinate indicates the temperature on a certain scale. In order to construct a phase diagram of alloys, first a series of cooling curves are constructed for alloys of the same elements with different concentrations.
A diagram is constructed based on these curves. Alloys, the components of which, upon solidification, form only mechanical mixtures, belong to the first group. The diagram of these alloys is conventionally called a phase diagram of the first kind. The diagram of alloys that form only solid solutions during solidification is called a phase diagram of the second kind. The most typical for diagrams of the first kind are alloys of lead and antimony.
Construction of a diagram (of the first kind) of the state of Pb-Sb alloys:
1) cooling curves of hypoeutectic alloys;
2) state diagram of Pb-Sb alloys;
3) cooling curves of hypereutectic alloys. The diagram is constructed for five types of lead-antimony alloy:
1) 5% antimony and 95% lead;
2) 10% antimony and 90% lead;
3) 20% antimony and 80% lead;
4) 40% antimony and 60% lead;
5) 80% antimony and 20% lead.
They all have two critical temperatures:
top and bottom. The study of the crystallization processes of these alloys shows that the upper critical temperature corresponds to the beginning, and the lower one to the end of solidification of the alloy. Thus, the process of crystallization of Pb-Sb alloys differs sharply from the crystallization of pure metals. Alloys crystallize over a temperature range, while pure metals crystallize at a constant temperature.
A mechanical mixture of crystals released from a liquid alloy simultaneously is called eutectic
(translated from Greek - “well-built”).
Alloys of the indicated concentration are called eutectic.
DIA
line on the diagram is called
the liquidus line
(translated from Greek as “liquid”).
Above this line, any lead-antimony alloy is in a liquid state. DSVE
line is called
the solidus
(translated from Greek as “solid”), or eutectic line.
Point C
shows the composition of the eutectic.
Alloys located to the left of this point are called hypoeutectic, and
those to the right are called
hypereutectic.
In the structure of hypoeutectic alloys, in addition to eutectic, there is always some amount of lead, and in hypereutectic alloys, in addition to eutectic, there is antimony.
Table of contents
Chemical properties of metals
Metals easily give up electrons, i.e. they are reducing agents. Therefore, they react easily with oxidizing agents.
Questions
- Which atoms are oxidizing agents?
- What are the names of simple substances consisting of atoms that are capable of accepting electrons?
Thus, metals react with non-metals. In such reactions, nonmetals, when accepting electrons, usually acquire a LOWER oxidation state.
Let's look at an example. Let aluminum react with sulfur:
Question. Which of these chemical elements is only capable of donating electrons? How many electrons?
Aluminum is a metal that has 3 electrons on its outer level (group III!), so it donates 3 electrons:
As the aluminum atom gives up electrons, the sulfur atom accepts them.
Question. How many electrons can a sulfur atom accept before completing the outer level? Why?
The sulfur atom has 6 electrons in its outer level (group VI!), therefore, this atom receives 2 electrons:
Thus, the resulting compound has the composition:
As a result, we obtain the reaction equation:
Task 8.5. Using similar reasoning, compose reaction equations:
- calcium + chlorine (Cl2);
- magnesium + nitrogen (N2).
When composing reaction equations, remember that a metal atom gives up all its external electrons, and a non-metal atom accepts as many electrons as there are missing up to eight.
The names of compounds obtained in such reactions always contain the suffix ID :
The root word in the name comes from the Latin name for a non-metal (see lesson 2.4).
Metals react with acid solutions (see lesson 2.2). When drawing up equations for such reactions and when determining the possibility of such a reaction, one should use a series of voltages (activity series) of metals:
Metals in this series before hydrogen are capable of displacing hydrogen from acid solutions:
Task 8.6. Write down equations for possible reactions:
- magnesium + sulfuric acid;
- nickel + hydrochloric acid;
- mercury + hydrochloric acid.
All these metals in the resulting compounds are divalent.
The reaction of a metal with an acid is possible if it results in a soluble salt. For example, magnesium practically does not react with phosphoric acid, since its surface is quickly covered with a layer of insoluble phosphate:
Metals after hydrogen can react with some acids, but hydrogen is not released in these reactions:
Task 8.7. Which of the metals - Ba, Mg, Fe, Pb, Cu - can react with a solution of sulfuric acid? Why? Write down equations for possible reactions.
Metals react with water if they are more active than iron (iron can also react with water). In this case, very active metals ( Li – Al ) react with water under normal conditions or with slight heating according to the following scheme:
where x is the valency of the metal.
Task 8.8. Draw up reaction equations according to this scheme for K, Na, Ca. What other metals can react with water in this way?
The question arises: why does aluminum practically not react with water? Indeed, we boil water in an aluminum pan, and... nothing! The fact is that the surface of aluminum is protected by an oxide film (relatively Al2O3). If it is destroyed, a reaction of aluminum with water will begin, and quite active. It is useful to know that this film is destroyed by chlorine ions Cl–. And since aluminum ions are unsafe for health, you should follow the rule: you cannot store highly salted foods in aluminum containers!
Question. Is it possible to store sour cabbage soup and compote in aluminum containers?
Less active metals, which are in the series of voltages after aluminum, react with water in a highly crushed state and with strong heating (above 100 °C) according to the following scheme:
Metals that are less active than iron do not react with water!
Metals react with salt solutions . In this case, more active metals displace the less active metal from the solution of its salt:
Task 8.9. Which of the following reactions are possible and why:
- silver + copper nitrate II;
- nickel + lead nitrate II;
- copper + mercury nitrate II;
- zinc + nickel nitrate II.
Write down equations for possible reactions. For impossible ones, explain why they are impossible.
It should be noted (!) that very active metals, which under normal conditions react with water, do not displace other metals from solutions of their salts, since they react with water and not with salt:
And then the resulting alkali reacts with salt:
Therefore, the reaction between ferrous sulfate and sodium is NOT accompanied by the displacement of the less active metal:
Methods for studying the structure of metals
macro- and microanalysis methods , the X-ray method , as well as flaw detection (X-ray, magnetic, ultrasonic).
The macroanalysis method is used to study the macrostructure , i.e. structure visible to the naked eye or with a magnifying glass. This reveals large defects: cracks, shrinkage cavities, gas bubbles, etc., as well as uneven distribution of impurities in the metal. The macrostructure is determined by the fractures of the metal, by macrosections (this is a sample of a metal or alloy, one of the sides of which is polished, thoroughly degreased, etched and examined with a magnifying glass with a magnification of 5–10 times).
Microanalysis reveals the structure of a metal or alloy using microsections, additionally polished to a mirror finish. The thin sections are examined in reflected light under an optical microscope at a magnification of up to 3000 times. Due to the different orientation of the metal grains, they are not etched to the same extent, and under a microscope, light is also reflected differently. Due to impurities, grain boundaries are etched more strongly than the base metal and are revealed more prominently. Knowing the microstructure, it is possible to explain the reasons for changes in the properties of the metal.
Using X-ray analysis, the atomic structure of metals, the types and parameters of crystal lattices, as well as defects lying in depth are studied. This analysis, based on the diffraction (reflection) of X-rays by rows of atoms in a crystal lattice, allows you to detect defects without destroying the metal. Instead of defects, X-rays are absorbed less than in solid metal, and therefore on photographic film such rays form dark spots corresponding to the shape of the defect.
The magnetic method is used to examine defects in magnetic metals (steel, nickel, etc.) at a depth of up to 2 mm. To do this, the product being tested is magnetized, its surface is coated with iron powder, the surface is inspected, and the product is demagnetized. A non-uniform field is formed around the defect, and the magnetic powder follows the contours of the defect. The ultrasonic method provides effective control of the quality of metal products and workpieces of almost any size. In pulsed ultrasonic flaw detectors, the ultrasonic wave from the probe-emitter propagates in the test product and, when it encounters a defect, is reflected from it. In this case, the reflected waves are received, amplified and transmitted to the indicating indicator.
Metal corrosion
Corrosion is a spontaneous process of metal oxidation under the influence of environmental factors.
Metals are practically not found in free form in nature. The only exceptions are “noble”, the most inactive metals, such as gold and platinum. All others are actively oxidized under the influence of oxygen, water, acids, etc. For example, rust forms on any unprotected iron product precisely in the presence of oxygen or water. In this case, iron is oxidized:
and the components of atmospheric moisture are restored:
As a result, iron ( II ) hydroxide is formed, which, when oxidized, turns into rust:
Other metals can also corrode, although rust does not form on their surface. So, there is no aluminum metal on Earth - the most common metal on the planet. But the basis of many rocks and soils is alumina Al2O3 . The fact is that aluminum instantly oxidizes in air. Metal corrosion causes enormous damage, destroying various metal structures.
To reduce losses from corrosion, the causes that cause it should be eliminated. First of all, metal objects should be insulated from moisture. This can be done in different ways, for example, storing the product in a dry place, which is not always possible. In addition, you can paint the surface of the object, lubricate it with a water-repellent composition, and create an artificial oxide film. In the latter case, chromium is introduced into the alloy, which “kindly” spreads its own oxide film over the surface of the entire metal. The steel becomes stainless.
Stainless steel products are expensive. Therefore, to protect against corrosion, they use the fact that the less active metal does not change, i.e., does not participate in the process. Therefore, if a more active metal is welded to the stored product, then until it collapses, the product will not corrode. This method of protection is called tread protection.
Main types of alloys
There are different types of metal alloys, but it is worth talking only about the main ones.
The most popular are iron-based formulations. These include steel, cast iron and ferrites. If everything is clear with the first two alloys, then it’s worth briefly talking about what ferrites are. These are metal compounds that contain large amounts of carbon. They are used to make inductors. Other major metal alloys are also worth mentioning.
Products made from metal alloys
Magnesium alloys
They have high strength with a small size and weight of the workpiece. They are poorly protected from corrosion and do not have sufficient ductility for easy processing. Used in mechanical engineering. The main feature of magnesium-based alloys is their ability to absorb vibrations of moving elements.
Beryllium alloys
Resistant to corrosive processes. Beryllium is most often mixed with copper. This mixture is called Beryllium Bronze. It is used for the manufacture of gears, contacts, clock mechanisms, and bearings.
Zinc alloys
The features of these compounds are their low melting point, high ductility, and corrosion resistance. Used for the manufacture of bearings, household appliances, and mechanical engineering.
Titanium alloys
Difficult to process material. Alloys based on it are lightweight, high strength, and resistant to environmental factors. To make metal processing easier, it must be heated. Used in various industries.
Aluminum alloys
Alloys based on this material are considered the most popular. You can meet them in most areas of human life. They have the following advantages:
- corrosion resistance;
- light weight;
- plastic;
- electrical conductivity.
The main disadvantage of this material is its low melting point. Already at 200 degrees, the properties of the alloy deteriorate. Aluminum alloys are used in various industries. Due to its low specific gravity, aluminum has become very popular in aircraft construction.
Aluminum (Photo: pixabay.com)
Copper alloys
Most copper-based compounds are brass. Depending on the copper content in the alloy, red and yellow brass are distinguished. Small parts for high-precision and miniature mechanisms are made from this material. It has a high ductility rate, making it easy to work with copper-based compounds.
conclusions
Metals are simple substances that are always reducing agents. The reduction activity of the metal decreases in the voltage series from lithium to gold. By the position of the metal in the stress series, you can determine how the metal reacts with acid solutions, with water, with salt solutions.
Lesson 9. Alkali and alkaline earth metals →
← Lesson 7. The concept of redox reactions
Want it even easier? We have created a new course where in a maximum of 7 days you will master chemistry from scratch. More details at the link
Hardness, fatigue, endurance
Hardness is the ability of a material to resist the penetration of another, harder body into it. Metal-cutting tools must have high hardness: cutters, drills, cutters, as well as surface-hardened parts. The hardness of the metal is determined by the Brinell , Rockwell and Vickers .
The measure of the hardness of the NV is taken as the ratio of the load to the surface area of the print with a diameter d and depth t, which is formed when a ball with a diameter D is pressed by force P. The numerical value of hardness is determined as follows: the diameter of the print is measured using an optical magnifying glass (with divisions), and from the resulting value is found in table attached to GOST, the corresponding hardness number. To assess the hardness of metals in small volumes, for example, on metal grains or its structural components, a method is used to determine microhardness .
Fatigue is the process of gradual accumulation of damage to a material under the influence of repeated alternating stresses, leading to the formation of cracks and destruction. Metal fatigue is caused by the concentration of stress in its individual volumes, in which there are non-metallic inclusions : gas bubbles, various local defects, etc. Characteristic is a fatigue fracture , which forms after the destruction of a sample as a result of repeated loading and consists of two parts that are different in appearance. One part of the fracture with a smooth (worn) surface is formed due to the friction of surfaces in the area of cracks arising from the action of repeatedly variable loads, the other part with a granular fracture occurs at the moment of destruction of the sample. Fatigue tests are carried out
on special machines. The most common machines are for repeated-alternating bending of a rotating sample, fixed at one or both ends, as well as machines for testing tension-compression and repeated-alternating torsion. As a result of the tests, the endurance limit is determined, which characterizes fatigue resistance.
Endurance is the property of a material to resist fatigue. The endurance limit is the maximum stress that a metal can withstand without failure for a specified number of loading cycles. There is an approximate relationship between the endurance limit and the strength limit
where σ-1 and σ-1р are the endurance limits for bending and tension-compression, respectively; σв is the tensile strength.