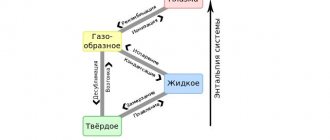
The fact that the properties of metals change when they are fused became known back in ancient times. 5 thousand years ago, our ancestors learned to make bronze - a mixture of tin and copper. Bronze is harder than both metals in its composition.
The properties of pure metals, as a rule, do not meet the necessary requirements, therefore, in almost all spheres of human activity, not pure metals, but their alloys are used. This is a material that is formed as a result of the solidification of the melt of two or more separate substances. In addition to metals, their composition may also include non-metals, for example, carbon or silicon.
By adding admixtures of other metals and non-metals in a certain amount, you can obtain many thousands of materials with a wide variety of properties, including those that none of the elements that make up the mixture have. Compared to the original metal, the alloy can be mechanically stronger and harder, with a significantly higher or lower melting point, more resistant to corrosion, more resistant to high temperatures, practically not change its dimensions when heated or cooled, etc.
Basic definitions
In general, metal alloys are materials obtained by smelting, in the production of which two or more metal elements (in the chemical sense) were used, as well as (optionally) special additives.
One of the first materials of this kind was bronze. It is composed of 85% copper and 15% tin (80:20 in the case of bell bronze). Currently, there are several varieties of this compound that do not contain tin at all. But they don't occur very often. It is necessary to clearly understand that metal alloys in most cases are formed without any human involvement at all. The fact is that it is possible to obtain a material that is absolutely pure from a chemical point of view only in the laboratory. Any metal that is used in everyday life probably contains traces of another element. A classic example is gold jewelry. Each of them contains a certain proportion of copper. However, in the classical sense, this definition still means a compound of two or more metals, which was purposefully obtained by man.
The entire history of man is an excellent example of how metal alloys were able to have a huge impact on the development of our entire civilization. It is no coincidence that there is even a long historical period called the “Bronze Age”.
Obtaining metals
In order to make an alloy, you must first obtain the metal from natural ore. Native elements are those substances that are found in nature in a free state. These include platinum, gold, tin, and mercury. They are separated from impurities mechanically or using chemical reagents.
Other metals are extracted by processing their compounds. They are found in various fossils. Ore is a mineral and rock containing metal compounds in the form of oxides, carbonates or sulfides. To obtain them, chemical processing is used.
Methods for obtaining metals:
• reduction of oxides with coal;
• obtaining tin from tin stone;
• smelting pig iron from iron ore;
• burning of sulfur compounds in special furnaces.
To facilitate the extraction of metals from ore rocks, various substances called fluxes are added to them. They help remove unwanted impurities such as clay, limestone, sand. As a result of this process, low-melting compounds called slag are obtained.
If there is a significant amount of impurities, the ore is enriched before smelting the metal by removing most of the unnecessary components. The most widely used methods of this processing are flotation, magnetic and gravitational methods.
General characteristics of metal alloys
Now we will look at the general properties of metals and alloys by which they are characterized. They can very often be found in specialized literature.
| Characteristic | Decoding |
| Strength | The ability of an alloy to withstand mechanical loads and resist destruction. |
| Hardness | A property that determines the resistance of a material to attempts to introduce a part from another alloy or metal into its thickness. |
| Elasticity | The ability to restore its original shape after the application of significant mechanical force or load. |
| Plastic | On the contrary, this is a property that characterizes the possibility of changing shape and size under the influence of applied force, mechanical load. In addition, this also characterizes the ability of a part to maintain its newly acquired shape for a long time. |
| Viscosity | - the ability of a metal to resist rapidly increasing (shock) loads |
These are the qualities that characterize metal alloys. The table will help you understand them.
Alloy types
There are different methods for producing metals, and the materials produced through them have different properties. In the solid state of aggregation, alloys are:
• Homogeneous (homogeneous), consisting of crystals of the same type. They are often called single-phase.
• Heterogeneous (non-uniform), called multiphase. When producing them, a solid solution (matrix phase) is taken as the basis of the alloy. The composition of heterogeneous substances of this type depends on the composition of its chemical elements. Such alloys may contain the following components: interstitial and substitutional solid solutions, chemical compounds (carbides, intermetallic compounds, nitrides), crystallites of simple substances.
Production information
In principle, at present, an “alloy” may well be understood as a material based on only one chemical element, but “diluted” with a whole package of additives.
The most common method of obtaining them, melting them to a liquid state, has changed little since ancient times. For example, analysis of metals and alloys shows that the ancient Indians mastered a level of metal processing that was amazing for their time. They even began to create alloys using refractory zinc, which in our time is a rather labor-intensive and complex procedure.
Today, powder metallurgy is also widely used for these purposes. Ferrous metals and alloys based on them are especially often processed by this method, since in this case the maximum cheapness of both the process itself and the manufactured product is often required.
Types of alloys
Despite such a variety of alloys, those based on iron and aluminum are of greatest importance to people. They are the ones that are most often encountered in everyday life. There are different types of alloys. Moreover, they are divided according to several criteria. Thus, various methods of producing alloys are used. According to this criterion they are divided into:
• Cast, which are obtained by melt crystallization of mixed components.
• Powder, created by pressing a mixture of powders and subsequent sintering at high temperature. Moreover, often the components of such alloys are not only simple chemical elements, but also their various compounds, such as titanium or tungsten carbides in hard alloys. Their addition in certain quantities changes the properties of metallic materials.
Methods for producing alloys in the form of a finished product or billet are divided into:
• foundries (silumin, cast iron);
• deformable (steel);
• powder (titanium, tungsten).
Distribution of alloys in modern industry
It should be noted that all metals that are intensively used by modern industry are alloys. Thus, more than 90% of all iron produced in the world is used for the production of cast iron and various steels. This approach to the matter is explained by the fact that metal alloys in most cases demonstrate better properties than their “progenitors”.
Thus, the yield strength of pure aluminum is only 35 MPa. But if you add 1.6% copper, magnesium and zinc to it in a ratio of 2.5% and 5.6%, respectively, then this figure can easily exceed even 500 MPa. Among other things, electrical conductivity, thermal conductivity, or other properties can be significantly improved. There is no mysticism in this: in alloys, the structure of the crystal lattice changes, which allows them to acquire other properties.
Simply put, the amount of this kind of material is large these days, but it is constantly growing.
Examples of problem solving
| Exercise | How many kg of tin must be added to a piece of bronze (m=4kg) containing 15% tin in order to increase the tin content in it to 60% of the total mass? |
| Solution | Let two alloys be mixed, the second alloy containing 100% tin and no other components. Let's find the masses of tin in the alloys: |
Then the mass of alloys will be:
The ratio of the mass of tin in the new alloy to the mass of the new alloy is:
Copper alloys are a compound of a non-ferrous metal with some elements of the periodic table. During their formation, the atoms of the copper crystal lattice are replaced by atoms of another substance. As a result, a new solid compound is formed. Each of them has its own physical and chemical characteristics.
Most often, bronze and brass are made from copper by adding zinc and tin. New connections reduce the price of the base metal, improving some parameters. There is an increase in ductility and corrosion resistance. This makes it possible to use them in some industries.
Become
All iron compounds containing up to 2% carbon are called steels.
If the composition contains chromium, vanadium or molybdenum, then they are called alloyed. We encounter these materials constantly, daily and hourly. The number of steels today is such that listing them alone could fill a not-too-thin book. We have already said that the mechanical properties of metals and alloys are very different, but in the case of these materials, even different types of steel often have opposite qualities, which is why their areas of application differ greatly.
If the material contains less than 0.25% carbon, then it is used in some technical structures. If the steel contains more than 0.55% carbon, it is ideal for the production of a variety of high-quality cutting tools, including lathe cutters, drills and surgical supplies. But if we are talking about devices that are used for fast cutting, then only alloy steel is used for their production.
Tool alloys
Designed for the manufacture of cutting tools, dies and parts of precision mechanisms. Such connections must be wear-resistant and durable, and their strength should not decrease significantly when heated. Such requirements are met, for example, by stainless steels that have undergone special processing (hardening).
Adding substances to alloys that improve their properties is called alloying. To impart the necessary properties, tool steels are usually alloyed with tungsten, vanadium or chromium.
Cast iron
If an iron alloy contains more than 3-4% carbon, then it is called cast iron. In addition, its important element is silicon. A lot of parts and finished products are made from cast iron. For example, engine blocks for cars. In the case of a high-quality casting without cavities or cavities, the product has impressive mechanical strength. In this regard, it is worth recalling at least the guns of the 14th-15th centuries, which often withstood a three to fourfold increase in the powder charge.
Of course, the use of metals and alloys was never limited exclusively to the military industry, but it often turned out that it was this branch of industry that constantly found new methods of metal processing, moving forward the entire civilization.
Methods of obtaining
The main methods for producing metals and alloys:
• Foundry, in which a homogeneous mixture of different molten components solidifies. To obtain alloys, pyrometallurgical and electrometallurgical methods for producing metals are used. In the first option, thermal energy obtained during fuel combustion is used to heat the raw materials. The pyrometallurgical method produces steel in open-hearth furnaces and cast iron in blast furnaces. In the electrometallurgical method, raw materials are heated in induction or electric arc furnaces. In this case, the raw materials are melted very quickly.
• Powder, in which powders of its components are used to make the alloy. Thanks to pressing, they are given a certain shape and then sintered in special ovens.
Copper alloys
Most often, this term refers to different types of brass. These are copper alloys that contain from 5 to 45% zinc. If its content ranges from 5-20%, then it is red brass (tompak). If the material already contains 20–36% Zn, then it is yellow brass.
These materials are ideal when it comes to producing and molding small parts. It is little known, but the alloy of copper and silicon is called silicon bronze and has great mechanical strength. The phosphorous variety is characterized by almost the same thing (5% tin and a certain amount of phosphorus are added to copper). As in the previous case, it is characterized by high strength and springy qualities, and therefore is ideal for the manufacture of membranes and various types of springs.
Alloys
Equipment requires a variety of metal materials. At the same time, pure chemical elements are practically not used, since they do not have the properties necessary for people. For our needs, we have invented different methods for producing alloys. This term refers to a macroscopically homogeneous material that consists of 2 or more chemical elements. In this case, metal components predominate in the alloy. This substance has its own structure. The following components are distinguished in alloys:
• base consisting of one or more metals;
• small additions of modifying and alloying elements;
• unremoved impurities (technological, natural, accidental).
Metal alloys are the main structural materials. There are more than 5,000 of them in technology.
Lead alloys
In general, non-ferrous metals and alloys are inseparably related concepts, since since ancient times people have been able to smelt multi-component materials, which they successfully used in military and peaceful affairs.
This especially applies to lead, from whose alloys the Romans made water pipes and sewerage systems. Of course, they did not know about the toxic properties of this metal, but they were impressed by the ease of its processing. The most commonly known solder at present is conventional solder, which is made from one part lead and two parts tin. As the name suggests, it is used for soldering parts. Used in radio engineering and other technical industries. Antimony and lead are used to make alloys that are used to make sheaths for various types of cables.
It has long been known that compounds of this metal with cadmium, bismuth or tin can melt at approximately 70 degrees Celsius. That is why today they are used to make various fuses in automatic fire extinguishing systems.
Oddly enough, lead has long been known to chefs and restaurateurs, as tableware and cutlery were often made from it. The alloy used for this is called pewter. It contains approximately 85–90% tin. The remaining 10-15% is occupied by lead (a standard alloy of two metals).
Technicians are also likely familiar with babbitts. These are also lead-based compounds that also contain tin, as well as arsenic and antimony. These alloys are very poisonous, but due to some special properties they are actively used in the bearing industry.
Application of low-melting alloys
The main required property is a specified low melting point. This property is, in particular, used for soldering microcircuits. In addition, these compounds must have a certain density, tensile strength, chemical inertness, and thermal conductivity.
Low-melting mixtures are made from bismuth, lead, cadmium, tin and other metals. Such alloys are used in temperature sensors, thermometers, fire alarms, for example, Wood's alloy. And also in foundry for the production of lost wax models, for fixation of bones and prosthetics in medicine. The sodium-potassium compound (melting point –12.5 °C) is used as a coolant for cooling nuclear reactors. Low-melting mixtures are used in foundry and are indispensable in fire alarm sensors
About light alloys
As we have already said, the properties of metals and alloys differ in that the latter in many cases have higher characteristics. This is especially noticeable in relation to modern industry. In recent years, it has required a huge amount of light alloys, which have increased mechanical strength, as well as resistance to adverse environmental factors and high temperatures.
Most often, aluminum, beryllium, and magnesium are used for their production. Compounds based on aluminum and magnesium are especially in demand, since the scope of their possible application is extremely wide.
Types of alloys
Various methods for producing alloys have allowed man to invent a large number of metal materials with different properties and characteristics. According to their purpose, they are divided into the following groups:
• Structural (steel, duralumin, cast iron). This group also includes alloys with special properties. So they differ in intrinsic safety or anti-friction properties. These include brass and bronze.
• For filling bearings (babbitt).
• For electric heating and measuring equipment (nichrome, manganin).
• For the production of cutting tools (will win).
In production, people also use other types of metal materials, such as fusible, heat-resistant, corrosion-resistant and amorphous alloys. Magnets and thermoelectrics (telurides and selenides of bismuth, lead, antimony and others) are also widely used.
Aluminum alloys
As we have already said, it is absolutely impossible to imagine modern industry without them. Judge for yourself: aluminum alloys are actively used in aviation, space, military, scientific and engineering and other industries. Without aluminum, it is impossible to imagine manufacturers of modern household and mobile appliances, since cases made of this metal are increasingly used by modern flagships of these industries.
Titanium alloys.
Titanium alloys are superior to both aluminum and magnesium alloys in terms of tensile strength and elastic modulus. Their density is greater than that of all other light alloys, but in terms of specific strength they are second only to beryllium. With a fairly low content of carbon, oxygen and nitrogen, they are quite plastic. The electrical conductivity and thermal conductivity of titanium alloys are low, they are resistant to wear and abrasion, and their fatigue strength is much higher than that of magnesium alloys. The creep limit of some titanium alloys at moderate stresses (about 90 MPa) remains satisfactory up to about 600 ° C, which is significantly higher than the temperature permissible for both aluminum and magnesium alloys. Titanium alloys are quite resistant to the action of hydroxides, salt solutions, nitric and some other active acids, but not very resistant to the action of hydrohalic, sulfuric and orthophosphoric acids.
Titanium alloys are forged up to temperatures of about 1150° C. They allow electric arc welding in an inert gas atmosphere (argon or helium), spot and roller (seam) welding. They are not very amenable to cutting (seizing of the cutting tool). Melting of titanium alloys must be done in a vacuum or controlled atmosphere to avoid contamination with oxygen or nitrogen impurities that cause embrittlement. Titanium alloys are used in the aviation and space industries for the manufacture of parts operating at elevated temperatures (150–430° C), as well as in some special-purpose chemical apparatus. Light armor for the cockpits of combat aircraft is made from titanium-vanadium alloys. Titanium-aluminum-vanadium alloy is the primary titanium alloy for jet engines and airframes.
In table Table 3 shows the characteristics of special alloys, and table. Figure 4 shows the main elements added to aluminum, magnesium and titanium, indicating the resulting properties.
Advantages and disadvantages of this material
Many alloys made from this material are economical, relatively inexpensive and very durable, as they do not corrode. They are distinguished by high strength in conditions of extremely low temperatures (aerospace industries) and a very simple processing process. Their molding does not require particularly complex and expensive equipment, since they are relatively plastic and viscous (see table with characteristics).
Unfortunately, they also have their drawbacks. Thus, at temperatures above 175 °C, the mechanical properties of aluminum and alloys based on it begin to rapidly deteriorate. But thanks to the presence of amalgam on their surface (a protective film of aluminum hydroxide), they have outstanding resistance to aggressive chemical environments, including acids and alkalis.
They have excellent electrical and thermal conductivity and are non-magnetic. It is believed that they are absolutely harmless to human health, and therefore they can be used for the production of food utensils and cutlery. However, recent medical researchers still say that aluminum compounds in some cases can provoke the development of Alzheimer's disease.
The military fell in love with these materials because they do not produce sparks even under sudden mechanical impacts and impacts. In addition, they perfectly absorb shock loads. Simply put, some of these metal alloys (the composition of which is most often classified) are actively used for the production of light armor to equip a variety of armored personnel carriers, infantry fighting vehicles, BRDMs and other equipment.
Thanks to all these properties, base alloys are widely used for the production of pistons for internal combustion engines, as well as in the production of building structures (corrosion resistance). Aluminum and materials based on it are widely used in the production of reflectors for lighting displays, electrical wiring, as well as for the manufacture of housings for various equipment (not magnetized).
It is important to note that even theoretically pure aluminum sometimes contains a significant admixture of iron. It can contribute to higher mechanical strength of the material, but its presence makes the aluminum-based alloy highly susceptible to corrosion processes. In addition, the alloy largely loses its ductility, which is also not very good in most cases.
Cobalt, chromium or manganese helps to weaken the negative effect of iron impurities. If the alloy contains lithium, the result is a very strong and elastic material. It is not surprising that this connection is very popular in the aerospace industry. Alas, lithium alloys with aluminum have an unpleasant property, which again manifests itself in poor ductility.
Let's summarize some results. It turns out that the main metal alloys in astronautics, aviation and other high-tech industries contain aluminum. In general, this is exactly how things stand today, but magnesium and its alloys are often used in modern industry.
Aluminum alloys.
These include casting alloys (Al – Si), die-casting alloys (Al – Mg) and self-hardening high-strength alloys (Al – Cu). Aluminum alloys are economical, easily accessible, strong at low temperatures and easy to process (they are easily forged, stamped, suitable for deep drawing, drawing, extruding, casting, well welded and machined on metal-cutting machines). Unfortunately, the mechanical properties of all aluminum alloys begin to deteriorate noticeably at temperatures above approximately 175° C. However, due to the formation of a protective oxide film, they exhibit good corrosion resistance in most common aggressive environments. These alloys conduct electricity and heat well, are highly reflective, non-magnetic, harmless in contact with food (since the corrosion products are colorless, tasteless and non-toxic), explosion-proof (since they do not produce sparks) and absorb shock loads well. Thanks to this combination of properties, aluminum alloys serve as good materials for lightweight pistons; they are used in carriage, automobile and aircraft construction, in the food industry, as architectural and finishing materials, in the production of lighting reflectors, technological and household cable ducts, and in the laying of high-voltage power lines.
The iron impurity, which is difficult to get rid of, increases the strength of aluminum at high temperatures, but reduces corrosion resistance and ductility at room temperature. Cobalt, chromium and manganese weaken the embrittling effect of iron and increase corrosion resistance. When lithium is added to aluminum, the elastic modulus and strength increase, making the alloy very attractive to the aerospace industry. Unfortunately, despite their excellent strength-to-weight ratio (specific strength), aluminum-lithium alloys have low ductility.
Magnesium alloys
They have extremely low weight and are also characterized by very impressive strength. In addition, these materials are excellent for the foundry industry, and the workpieces are perfectly amenable to turning and milling. Therefore, they are actively used in the production of missiles and aircraft turbines, instrument housings, car wheel rims, as well as some types of armor steel.
Some varieties of these alloys are distinguished by excellent viscous damping properties, and therefore they are used for the production of parts and structures that have to work under conditions of extremely high levels of vibration.
Iron alloys
Almost all the iron smelted on Earth is used to produce simple and alloy steels. It is also used in the production of cast iron. Iron alloys gained their popularity due to the fact that they have properties beneficial to humans. They were obtained by adding various components to a simple chemical element. So, despite the fact that various iron alloys are made on the basis of the same substance, steel and cast iron have different properties. Thanks to this, they find different areas of application. Most steels are harder than cast iron. Different methods for obtaining these metals make it possible to obtain different grades (grades) of these iron alloys.
Advantages and disadvantages of magnesium alloys
They are quite soft, resist wear relatively well, but are not very impressive in their ductility. But they are distinguished by their excellent adaptability to forming at high temperatures, are perfectly suited for joining using all existing types of welding, and can also be connected by bolting, riveting and even gluing.
Alas, all these alloys are not particularly resistant to acids and alkalis. They are extremely negatively affected by prolonged exposure to sea water. However, magnesium alloys are surprisingly stable in air conditions, so many of their disadvantages can be neglected. If it is necessary to reliably protect such parts from corrosion, then chrome plating, anodizing or similar methods are used.
They can be clad with nickel, copper or chromium, after first immersing them in a melt of chemically pure zinc. With this treatment, their strength and abrasion resistance increase sharply. It must be recalled that magnesium is a fairly active metal from a chemical point of view, and therefore when working with it it is necessary to observe at least basic safety measures.
Thus, the production of metals and alloys is a key feature of modern industry. Every year people are inventing more and more ways to obtain new materials, so soon we will probably get absolutely incredible compounds that will combine the beneficial properties of several groups of materials and chemical elements at once.
Properties of alloys
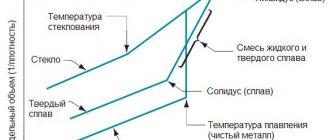
The properties of alloys depend on their structure. Alloys are characterized by structure-insensitive (determined by the nature and concentration of the elements that make up the alloys) and structure-sensitive properties (depending on the characteristics of the base). The structurally insensitive properties of alloys include density, melting point, and heat of evaporation. thermal and elastic properties, coefficient of thermal expansion.
All alloys exhibit properties characteristic of metals: metallic luster, electrical and thermal conductivity, ductility, etc.
Also, all the properties characteristic of alloys can be divided into chemical (the relationship of alloys to the effects of active media - water, air, acids, etc.) and mechanical (the relationship of alloys to the effects of external forces). If the chemical properties of alloys are determined by placing the alloy in an aggressive environment, then special tests are used to determine the mechanical properties. So, to determine strength, hardness, elasticity, ductility and other mechanical properties, tensile, creep, impact strength, etc. tests are carried out.
Historical perspective
According to historical data, the first copper alloy appeared by 7 thousand BC. Later, tin was used as an additive. At this time, called the Bronze Age, weapons, mirrors, dishes and jewelry were made from such material.
Read also: Wiring diagram for the motor from the washing machine
Production technology has changed. Additives appeared in the form of arsenic, lead, zinc and iron. Everything depended on the requirements for the subject. The material for jewelry required a special approach. The alloy composition consisted of copper, tin and lead.
Since the 8th century. BC e. In Asia Minor, the technology for producing brass was developed. At this time, they had not yet learned how to mine pure zinc. Therefore, its ore was used as a raw material. Over time, the production of copper alloys has constantly expanded and is still at the forefront.
Physical and chemical properties of alloys
The chemical composition and mechanical properties of copper alloys provide them not only with strength, but also with good electrical and thermal conductivity. This especially applies to brass.
All copper alloys are characterized by good anti-friction properties. Bronze is especially worth noting.
Due to the good anti-friction properties of bronze, the material is used for the manufacture of bushings as sliding bearings. Such a product does not require lubrication, since all roughness is crushed from the inner diameter along which sliding occurs. This is precisely the source of lubrication. Installation of such bearings is carried out even on high-precision equipment - jig boring and jig grinding machines.
The melting point of copper without additives is 1083 degrees. Depending on the amount of zinc and tin added, this indicator changes. The melting point of brass is 900–1050 degrees, and that of bronze is 930–1140 degrees.
Read also: How to weld cast iron with an electrode
The corrosion properties of copper alloys are resistant. This is due to the fact that copper is not an active element. Polished surfaces are especially non-corrosive.
The corrosion resistance of copper compounds occurs in fresh water and is impaired in the presence of acid, which prevents the formation of a protective coating.