Ferrous and non-ferrous metals
Metals are chemically simple substances characterized by good luster, high thermal and electrical conductivity, opacity, and fusibility.
Some of the metals can be forged and welded. Black metals include iron and alloys based on it - cast iron and steel , as well as ferroalloys . The remaining metals make up the non-ferrous . Of the non-ferrous metals, the most important industrial ones are copper, aluminum, lead, tin, nickel, titanium, etc. Non-ferrous metals have a number of physical and chemical properties that make them indispensable in technology. Currently, rare non-ferrous metals : gallium, indium, beryllium, cerium, cesium, neodymium and others, which have very high physicochemical and mechanical properties, both in pure form and in compounds with other metals. Gallium , having a low melting point (29.8°C), boils at a temperature of 2230°C; it is widely used to make thermometers designed to measure high temperatures.
Indium is highly reflective, scattering light evenly and is used to make mirrors and spotlights. A thin layer of indium protects windshields from icing.
Beryllium is the strongest of all light metals. Its density is 1.84 g/cm3, it is 1.5 times lighter than aluminum, and its specific strength is five times greater than aluminum, and titanium three times. Beryllium has high acoustic properties. The speed of sound in it travels 2.5 times faster than in steel. Beryllium is necessary for use in nuclear technology. Beryllium bronze is used in mechanical engineering and other industries.
Lithium is used in nuclear engineering and radio engineering. Lithium oxide-based lubricant does not freeze at temperatures of –50°C.
Niobium has high acid resistance, it is extremely ductile, it can be processed in the cold, the melting point of niobium is 2500°C. The central part of a nuclear reactor is made from an alloy of niobium and cesium.
Tantalum is a refractory metal, its melting point is 2996°C, it is corrosion-resistant, and is used in the form of plates and wires in bone and plastic surgery.
Osmium is one of the heaviest and hardest metals, very wear-resistant, used for the manufacture of surgical instruments, gold-plated nibs in fountain pens, long-lasting needles, axes and supports of precision measuring instruments and clock mechanisms.
What is a metal and how does it differ from a non-metal?
In other words, how can we understand that there is a metallic material in front of us? The answers to all these questions can be obtained by considering the unique properties of metals. These include the following main ones:
- The presence of a metallic sheen when polishing the surface. All metals shine, most of them are gray in color, however, some metals have a specific color, for example, bismuth is pink, copper is reddish, and gold is yellow.
- High thermal conductivity and electrical conductivity. At room temperature, the highest values for these physical properties are characteristic of copper and silver.
- At room temperature, almost all metals are in a solid state of matter. The exception is mercury, which melts already at -39 oC.
- Being in a solid state, metals are characterized by a crystalline structure. If the melt of the material in question is cooled too quickly, it acquires an amorphous structure, in which short-range order is still preserved.
- The melting points and densities of metals vary widely. Thus, the element tungsten is the most refractory (3410 oC). The heaviest is osmium (22.6 times denser than water), and the lightest is lithium (almost 2 times less dense than water).
- All metals are chemically active. Since they have low electronegativity, in chemical reactions their atoms give up electrons and turn into positively charged ions (cations).
Above in the list were listed the main properties of metals that distinguish them from non-metallic materials. Examples of the latter are oxygen, nitrogen, noble gases, sulfur, silicon, carbon and some others. Note that all living organisms consist mainly of non-metals.
Types of crystal lattices
A crystal lattice is an imaginary spatial grid, at the nodes of which atoms (ions) forming a metal are located. The particles of matter (ions, atoms) from which the crystal is built are located in a certain geometric order, which is periodically repeated in space.
In amorphous bodies (glass, plastics), unlike crystals, atoms or molecules are arranged randomly and chaotically.
The formation of a crystal lattice in a metal occurs as follows:
- when a metal transitions from a liquid to a solid state, the distance between the atoms decreases, and the interaction forces between them increase;
- when atoms come closer, electrons located on the outer shells lose contact with their atoms due to the removal of the valence electron of one atom by the positively charged nucleus of another, etc.;
- free electrons are formed, since they do not belong to individual atoms.
Thus, in the solid state, a metal is a structure consisting of positively charged ions around which free electrons move. Bonding in metal is carried out by electrostatic forces . Electrostatic attractive forces arise between the ions and free electrons, which “pull” the ions together. Such a bond between metal particles is called metallic .
Bonding forces in metals are determined by repulsive forces and attractive forces between ions and electrons. The ions are located at such a distance from one another at which the potential interaction energy is minimal. In a metal, ions are arranged in a certain order, forming a crystal lattice. This arrangement of ions is ensured by their interaction with valence electrons, which bind the ions in the crystal lattice. The types of crystal lattices are different for different metals. The most common lattices are: body-centered cubic (bcc) - -Fe, Cr, W, face-centered cubic (fcc) - -Fe, Al, Cu and hexagonal close-packed (hcp) - Mg, Zn, etc.
The smallest volume of a crystal, which gives an idea of the atomic structure of the metal in any volume, is called the elementary crystalline cell.
Internal structure and physical properties of metals
Metals are simple substances whose atoms can only give up electrons. This feature of metals is due to the fact that at the outer level of these atoms there are few electrons (most often from 1 to 3) or the outer electrons are located far from the nucleus. The fewer electrons at the outer level of the atom and the further they are located from the nucleus, the more active the metal (the more pronounced its metallic properties).
Task 8.1. Which metal is more active:
Name the chemical elements A, B, C, D.
Metals and non-metals in Mendeleev's Periodic Table of Chemical Elements (PSM) are separated by a line drawn from boron to astatine. Above this line in the main subgroups are non-metals (see lesson 3). The remaining chemical elements are metals.
Task 8.2. Which of the following elements are metals: silicon, lead, antimony, arsenic, selenium, chromium, polonium?
Question. How can we explain the fact that silicon is a non-metal, and lead is a metal, although they have the same number of outer electrons?
An essential feature of metal atoms is their large radius and the presence of valence electrons weakly bound to the nucleus. For such atoms, the ionization energy* is small.
* IONIZATION ENERGY is equal to the work spent on removing one external electron from an atom (to ionize an atom) that is in the ground energy state.
Some of the valence electrons of metals, breaking away from atoms, become “free”. “Free” electrons easily move between atoms and metal ions in the crystal, forming an “electron gas” (Fig. 28).
At a subsequent moment in time, any of the “free” electrons can be attracted by any cation, and any metal atom can give up an electron and turn into an ion (these processes are shown in Fig. 28 by dotted lines).
Thus, the internal structure of a metal is similar to a layer cake, where positively charged “layers” of metal atoms and ions alternate with electronic “layers” and are attracted to them. The best model of the internal structure of a metal is a stack of glass plates moistened with water: it is very difficult to tear one plate from another (strong metals), and it is very easy to move one plate relative to another (ductile metals) (Fig. 29).
Task 8.3. Make such a “model” of the metal and verify these properties.
A chemical bond carried out by “free” electrons is called a metallic bond .
“Free” electrons also provide such physical properties of metals as electrical and thermal conductivity, plasticity (malleability), as well as metallic luster.
Task 8.4. Find metal objects around the house.
By completing this task, you can easily find metal utensils in the kitchen: pots, pans, forks, spoons. Machine tools, airplanes, cars, diesel locomotives, and tools are made from metals and their alloys. Modern civilization is impossible without metals, since electrical wires are also made of metals - Cu and Al. Only metals are suitable for making antennas for radio and television receivers; the best mirrors are made from metals. In this case, not pure metals are often used, but their mixtures (solid solutions) - ALLOYS.
Defects in crystals
Crystals always have structural defects caused by a violation of the arrangement of atoms in the crystal lattice. Defects in the crystal structure are divided into point , linear and surface .
The reason for the formation of defects is vacancies (the place where an atom with higher energy was located and moved from one place to another). After some time, one of the atoms of the neighboring layer moves to this vacancy site, etc. Thus, the vacancy moves deeper into the crystal. With increasing temperature, the number of vacancies increases, and they move more often from one site to another. Point defects also include an atom embedded in an interstitial site of a crystal lattice, and a substituted atom, when the place of an atom of one metal is replaced in the crystal lattice by another, foreign atom. Point defects cause local distortion of the crystal lattice. Linear defects are another important type of imperfection of a crystal lattice, when, as a result of a shift of one interatomic distance of one part of the lattice relative to another along a certain plane, the number of rows of atoms in the upper part of the lattice is one more than in the lower part. In this case, an extra atomic plane ( extraplane ) appeared in the upper part of the lattice. The edge of the extraplane, perpendicular to the shear direction, is called edge (linear) dislocation , the length of which can reach many thousands of interatomic distances. The crystal lattice in the dislocation zone is elastically distorted, since the atoms in this zone are displaced relative to their equilibrium state.
Surface defects represent interfaces between individual crystals. At the interface, the atoms of the crystal are not as ordered as in its bulk. In addition, dislocations and vacancies accumulate along the interfaces, and impurities are concentrated, which further disrupts the arrangement of atoms. In this case, the crystals themselves are misoriented, i.e. can be rotated relative to each other by tens of degrees.
Defects in crystals significantly affect the properties of metals. To obtain high-quality metals and alloys, it is necessary to reduce all defects to a minimum. This can be achieved by resorting to special processing methods, such as heat treatment methods.
structure
Properties of refractory metals and alloys
The top image shows some emerald gemstones. Likewise, many other minerals, salts, metals, alloys and diamonds have a crystalline structure; But what is the relationship between its order and symmetry??
If symmetry operations (invert it, rotate it at different angles, reflect it in a plane, etc.) are applied to a crystal whose particles can be observed with the naked eye, it will be found to remain intact in all dimensions of space.
The opposite is true for an amorphous solid, from which various orderings are obtained by subjecting it to symmetry operations. Additionally, it lacks structural patterns of repetition, as demonstrated by the random distribution of its particles.
What is the smallest unit that makes up a structural pattern? In the top image, a crystalline solid is symmetrical in space, but an amorphous one is not.
If you draw some squares that surround the orange spheres and apply symmetry operations, you will find that they generate other parts of the crystal.
The previous thing is repeated with smaller and smaller squares until an asymmetrical one is found; preceding it in size is, by definition, a unit cell.
Unitary cell
A unitary cell is the minimum structural expression that allows the complete reproduction of a crystalline solid. From this you can assemble a crystal by moving it in all directions of space.
It can be thought of as a small box (chest, bucket, container, etc.) in which particles, represented by spheres, are placed in a fill pattern. The dimensions and geometry of this box depend on the length of its axes (a, b and c), as well as on the angles between them (α, β and γ).
The simplest of all unit cells is the simple cubic structure (top image (1)). In this case, the center of the spheres occupies the corners of the cube, placing four at its base and four on the roof.
With this arrangement, the spheres barely occupy 52% of the total volume of the cube, and since nature abhors a vacuum, there are not many compounds or elements that adopt this structure.
However, if the spheres are arranged in the same cube in such a way that it occupies the center (cubic center on the body, bcc), then a more compact and efficient packing will be available (2). Now spheres occupy 68% of the total volume..
On the other hand, in (3) no sphere occupies the center of the cube, but the center of their faces, and they all occupy up to 74% of the total volume (cubic center on the faces, cc).
Thus, it can be seen that for the same cube other schemes can be obtained by varying the way the spheres are packed (ions, molecules, atoms, etc.).
Anisotropy of crystals
The behavior and properties of crystals are influenced by many internal and external factors. Anisotropy refers to the difference in the physical properties of a medium in different directions. The following properties differ:
- strength;
- hardness;
- electrical resistance;
- thermal expansion.
The reason for anisotropy is the difference in the packing density of atoms or molecules in a lattice in different directions.
All crystals have the property of anisotropy, and amorphous bodies (glass, resin, rubber, paraffin, etc.) are isotropic , i.e. have the same atomic density in different directions.
Anisotropy of properties is important when using single crystals —single crystals whose particles are uniformly distributed throughout their volume. Single crystals have a regular crystalline cut (in the form of natural polyhedra) and are anisotropic in mechanical, electrical and other physical properties. Thus, for a copper single crystal, the tensile strength v varies from 120 to 360 MPa depending on the direction of application of the load. And a single crystal of table salt can collapse if you apply the slightest force to one of its sides.
Metals and alloys used in technology usually have a polycrystalline structure , i.e. consist of many small and differently oriented crystals that do not have a regular crystalline cut and are called crystallites (or grains). Anisotropy is observed in polycrystals. But due to the varied, random orientation of crystallographic planes in different grains, a polycrystal can have the same or similar properties in different directions and not exhibit anisotropy (when the grain sizes are significantly smaller than the sizes of the polycrystal and their number is very large). Therefore, a polycrystalline body is often considered to be similar to an isotropic one . But when working more delicately with alloys that have a polycrystalline structure, it is necessary to take into account their anisotropy. It can be caused by changes in external conditions (temperature, pressure, etc.) or the presence of foreign impurities in the material.
Features of the structure of solid and liquid bodies
Composition, structure and properties of metal alloys
Before answering the question of what melting is, we should consider the structural features of solid and liquid bodies.
The former are characterized by the presence of a constant form, any change of which they resist. Solids have elasticity and lack of fluidity. The distances between the particles forming a solid are small, and the bonding forces between these particles are significant compared to those for liquids and gases. Bonding forces in solids can have a different chemical nature (van der Waals, metallic, covalent, ionic). There are two ways to organize solids:
- crystalline structures, when the atoms or molecules of a body are located in certain positions in space, for example, metals;
- amorphous structures in which atoms or molecules are arranged in a chaotic manner, such as glass.
In liquids, atoms and molecules are further apart than in solids, so they are less strongly bonded. The liquid retains volume under these conditions, but does not retain its shape and has good fluidity. Liquid particles are located randomly relative to each other.
An important point to note is that atoms or molecules in a solid are in certain positions, which they change very slowly (for example, in diffusion processes), but liquid particles constantly jump from one position to another.
Crystallization of metals
The transition from a liquid to a solid (crystalline) state is called crystallization .
Crystallization processes depend on temperature and occur over time, therefore cooling curves are plotted in temperature-time coordinates (Fig. 1).
Rice. 1
The ideal process of metal crystallization without supercooling occurs at temperature TS. When the ideal solidification temperature TS is reached, the temperature drop stops. Each pure metal crystallizes at a strictly individual constant temperature. The purer the liquid metal, the more prone it is to hypothermia. As the cooling rate increases, the degree of supercooling increases, and the metal grains become smaller, which improves its quality. For most metals, the degree of supercooling during crystallization under production conditions ranges from 10 to 30°C.
The crystallization process occurs in two stages:
- nucleation of crystals (nuclei, or crystallization centers);
- growth of crystals from centers.
When the alloy is supercooled below the temperature TN, crystalline nuclei :
- the resulting crystals grow freely and have a regular geometric shape;
- when growing crystals come into contact, their correct shape is disrupted, since in these areas the growth of the faces stops;
- crystal growth continues in those directions where there is free access to liquid metal;
- crystals that initially had a geometrically regular shape, after solidification, acquire an irregular shape (they are called crystallites, or grains).
The size of grains formed during crystallization depends not only on the number of spontaneously nucleating crystallization centers, but also on the amount of insoluble impurities always present in the liquid metal. They are centers of crystallization . The crystal lattice of such solid particles should be close in structure and lattice parameters to the crystallizing metal. The formation of crystallization centers is also affected by the cooling rate.
Electronic structure of metals and their features
The internal structure of real metals determines their physical and chemical parameters.
Crystal lattice of metals
All metals in the solid phase have a crystalline structure. This spatial formation of repeatedly repeating primary structures is called a crystal lattice. crystal lattice diagram.
Crystal structure of metals
The crystal structure of metals and alloys can be of two types:
- The interatomic distance is equal in all directions. This is the so-called isotropic structure. Moreover, the physical properties of the crystal are also the same in all directions.
- The interatomic distance horizontally and vertically is different. Such a crystal is called anisotropic; its parameters depend on the direction.
In a real piece of metal, which consists of many crystalline fragments, the atomic-crystalline structure belongs to the third type - quasi-isotropic. The averaged parameters of such a piece are close to isotropic.
Structure of a mechanical ingot
The shape of the growing crystals is determined by:
- the conditions of their contact with each other;
- alloy composition;
- presence of impurities;
- cooling mode.
The mechanism of crystal formation is dendritic (tree-like) in nature. Dendritic crystallization is characterized by the fact that the growth of nuclei occurs at an uneven rate. After the formation of nuclei, their development proceeds in those planes and lattice directions that have the highest packing density of atoms and the minimum distance between them. In these directions, long branches of the future crystal are formed - first-order axes . axes of the second order begin to grow , from the axes of the second order - axes of the third order , etc.
Steel ingots are produced by cooling in metal molds ( molds ) or in continuous casting plants. In a mold, steel cannot harden simultaneously throughout its entire volume, since it is impossible to create a uniform rate of heat removal. Therefore, the process of steel crystallization begins at the cold walls and bottom of the mold and spreads into the liquid metal. When the liquid metal comes into contact with the walls of the mold, a zone of small equiaxed crystals . Since the volume of solid metal is less than liquid, an air gap and the wall itself heats up from contact with the metal, so the cooling rate of the metal decreases and the crystals grow in the direction of heat removal. In this case, a zone is formed consisting of tree-like (columnar) crystals .
In the inner zone of the ingot, as a result of slow cooling, equiaxed, non-oriented crystals of large sizes are formed. In the upper part of the ingot, which solidifies last, a shrinkage cavity , since upon cooling the volume of the metal decreases. Under the shrinkage cavity, the metal turns out to be loose due to the large number of shrinkage pores.
To obtain products, only part of the ingot is used, removing the shrinkage cavity and loose metal of the ingot for subsequent remelting.
Methods for studying the structure of metals
macro- and microanalysis methods , the X-ray method , as well as flaw detection (X-ray, magnetic, ultrasonic).
The macroanalysis method is used to study the macrostructure , i.e. structure visible to the naked eye or with a magnifying glass. This reveals large defects: cracks, shrinkage cavities, gas bubbles, etc., as well as uneven distribution of impurities in the metal. The macrostructure is determined by the fractures of the metal, by macrosections (this is a sample of a metal or alloy, one of the sides of which is polished, thoroughly degreased, etched and examined with a magnifying glass with a magnification of 5–10 times).
Microanalysis reveals the structure of a metal or alloy using microsections, additionally polished to a mirror finish. The thin sections are examined in reflected light under an optical microscope at a magnification of up to 3000 times. Due to the different orientation of the metal grains, they are not etched to the same extent, and under a microscope, light is also reflected differently. Due to impurities, grain boundaries are etched more strongly than the base metal and are revealed more prominently. Knowing the microstructure, it is possible to explain the reasons for changes in the properties of the metal.
Using X-ray analysis, the atomic structure of metals, the types and parameters of crystal lattices, as well as defects lying in depth are studied. This analysis, based on the diffraction (reflection) of X-rays by rows of atoms in a crystal lattice, allows you to detect defects without destroying the metal. Instead of defects, X-rays are absorbed less than in solid metal, and therefore on photographic film such rays form dark spots corresponding to the shape of the defect.
The magnetic method is used to examine defects in magnetic metals (steel, nickel, etc.) at a depth of up to 2 mm. To do this, the product being tested is magnetized, its surface is coated with iron powder, the surface is inspected, and the product is demagnetized. A non-uniform field is formed around the defect, and the magnetic powder follows the contours of the defect. The ultrasonic method provides effective control of the quality of metal products and workpieces of almost any size. In pulsed ultrasonic flaw detectors, the ultrasonic wave from the probe-emitter propagates in the test product and, when it encounters a defect, is reflected from it. In this case, the reflected waves are received, amplified and transmitted to the indicating indicator.
Structure and structure of metals
CHAPTER 7
METALS IN CONSTRUCTION
General information about metals. Metal classification
Metals (from Latin metallum - mine, mine) are a group of elements in the form of simple substances that have characteristic metallic properties, such as high thermal and electrical conductivity, positive temperature coefficient of resistance, high ductility and metallic luster.
Most metals are present in nature in the form of ores and compounds. They form oxides, sulfides, carbonates and other chemical compounds. To obtain pure metals and their further use, it is necessary to isolate them from ores and carry out purification. If necessary, alloying and other processing of metals is carried out. The science of metallurgy studies this. Metallurgy distinguishes ores of ferrous metals (based on iron) and non-ferrous (they do not contain iron, about 70 elements in total).
All metals and alloys are divided into two groups: ferrous metals and non-ferrous metals.
Ferrous metals are an alloy of iron with a small amount of carbon. Along with carbon, ferrous metals may contain silicon, manganese, phosphorus, sulfur and other chemical elements that enter the metals from ores or are added to them during the smelting process. To improve quality or impart specific properties, alloying additives are introduced into the composition of ferrous metals - copper, nickel, chromium, silicon.
Depending on the carbon content, ferrous metals are divided into cast iron and steel.
Steels contain up to 2% carbon, and cast irons contain from 2 to 6.7% carbon.
Non-ferrous metals are alloys based on aluminum, magnesium, copper, nickel, chromium, zinc, tin, and lead.
Metals consist of grains closely adjacent to each other. These grains can be seen on a fresh fracture of a metal rod with the naked eye. The structure of the metal is more clearly visible under a microscope at high magnification.
Depending on the chemical composition, the structural components of iron-carbon alloys have the following names:
— austenite
— a solid solution of carbon in gamma iron (gamma iron is one of the forms of pure iron crystals); limiting carbon concentration in austenite 1.7%; austenite is non-magnetic, characterized by high viscosity, good abrasion resistance and chemical resistance;
— ferrite
- technically pure iron, which is characterized by low hardness, low strength and high ductility; ferrite is magnetic; the properties of ferrite largely depend on the size of its grain; in the structure of steel, ferrite is located in the form of individual light grains interspersed with dark areas of pearlite, or in the form of light borders around pearlite grains;
— cementite
- a chemical compound of iron and carbon; has high hardness, but at the same time is fragile; the form of cementite in steel affects its mechanical properties, especially impact strength;
— perlite
— a mixture of cementite and ferrite; the carbon content in perlite is 0.83%; the finer the perlite grains in the metal, the higher its mechanical properties.
The chemical composition and structure of the metal determines its physical and mechanical properties: strength, hardness, density. Mechanical properties largely determine how well a part will perform under operating conditions.
Structure and structure of metals
Metals are solids with a crystalline structure.
A solid is a state of aggregation of a substance, characterized by stability of shape and volume. Based on their internal structure, solids are divided into crystalline and amorphous.
Crystals are solid bodies whose particles are arranged in a strict order, forming spatial periodically repeating structures.
More precisely, particles oscillate around certain equilibrium positions. If you mentally connect them with straight lines, you get a kind of “skeleton” of the crystal. This image of a crystal is called a crystal lattice.
It has been theoretically proven that a total of 230 different spatial crystal structures can exist.
Most of them (but not all) are found in nature or created artificially.
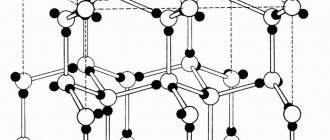
Rice. 7.1. Types of crystal structure of metals
In Fig. 7.1. examples of simple crystal lattices are given: 1 – simple cubic lattice; 2 – face-centered cubic lattice; 3 – body-centered cubic lattice; 4 – hexagonal lattice.
Metals have relatively complex types of cubic lattices—body-centered (bcc) and face-centered (fcc) cubic lattices.
Rice. 7.2. Body-centered crystal lattice
The basis of the bcc lattice is an elementary cubic cell (Fig. 7.2.), in which positively charged metal ions are located at the vertices of the cube, and another atom in the center of its volume, i.e. at the intersection of its diagonals. Iron, chromium, vanadium, tungsten, molybdenum and other metals have this type of lattice in certain temperature ranges.
Fig.7.3. Face centered crystal lattice
In a face-centered crystal lattice (fcc lattice) (Fig. 7.3), the unit cell is a cube with centered faces. Iron, aluminum, copper, nickel, lead and other metals have a similar lattice.
Fig.7.4. Hexagonal close-packed crystal lattice
The third common type of close-packed lattices is hexagonal close-packed (HCP, Fig. 7.4). The hcp cell consists of parallel centered hexagonal bases spaced from each other by a parameter. Three ions (atoms) are located on the middle plane between the bases.
In hexagonal lattices, the ratio of the parameter c/a is always greater than unity. Magnesium, zinc, cadmium, beryllium, titanium, etc. have such a lattice.
The compactness of the crystal lattice or the degree to which its volume is filled with atoms is an important characteristic. It is determined by such indicators as the lattice parameter, the number of atoms in each unit cell, the coordination number and packing density.
The lattice parameter is the distance between atoms along the edge of a unit cell. Lattice parameters are measured in nanometers (1 nm = 10-9 m = 10 Å). The parameters of cubic lattices are characterized by the length of the edge of the cube and are denoted by the letter a .
To characterize a hexagonal lattice, two parameters are taken - the side of the hexagon a and the height of the prism c . When the ratio c/a = 1.633, then the atoms are most densely packed, and the lattice is called hexagonal close-packed (Fig. 7.4). Some metals have a hexagonal lattice with a less dense packing of atoms ( c/a > 1.633). For example, for zinc c/a = 1.86, for cadmium c/a = 1.88.
The parameters of cubic metal lattices range from 0.286 to 0.607 nm. For metals with a hexagonal lattice, a lies in the range of 0.228-0.398 nm, and c in the range of 0.357-0.652 nm.
The crystal lattice parameters of metals can be measured using X-ray diffraction analysis.
When calculating the number of atoms in each unit cell, it should be kept in mind that each atom is simultaneously included in several cells. For example, for an fcc lattice, each atom located at the vertex of a cube belongs to 8 cells, and the atom centering the face belongs to two. And only the atom located in the center of the cube completely belongs to this cell.
Thus, bcc and fcc cells contain 2 and 4 atoms, respectively.
The coordination number is the number of nearest neighbors of a given atom.
Physical and chemical properties of metals
The physical properties of metals include color, density, melting point, thermal conductivity, thermal expansion, heat capacity, electrical conductivity, magnetic properties, etc.
Color refers to the ability of metals to reflect light of a certain wavelength. For example, copper is pink-red in color, aluminum is silver-white. The density of a metal is characterized by its mass contained in a unit volume. Based on density, all metals are divided into light (less than 4500 kg/m3) and heavy. Density is of great importance when creating various products. For example, in aircraft and rocket production they tend to use lighter metals and alloys (aluminum, magnesium, titanium), which helps reduce the weight of products.
Melting point is the temperature at which a metal changes from solid to liquid. Based on their melting point, they distinguish between refractory metals (tungsten - 3416°C, tantalum - 2950°C, titanium -1725°C, etc.) and low-melting metals (tin - 232°C, lead - 327°C, zinc - 419.5°C , aluminum - 660°C). Melting point is of great importance when choosing metals for the manufacture of cast products, welded and soldered joints, thermoelectric devices and other products. In SI units, the melting point is expressed in degrees Kelvin (K).
Thermal conductivity is the ability of metals to transfer heat from hotter to cooler areas of the body. Silver, copper, and aluminum have high thermal conductivity. Iron has a thermal conductivity about three times less than aluminum and five times less than copper. In SI units, thermal conductivity has the dimension W/ (m K).
Thermal expansion is the ability of metals to increase in size when heated and decrease when cooled. Thermal expansion is characterized by the coefficient of linear expansion
where l1 and l2 are the lengths of the body at temperatures t1 and t2. The coefficient of volumetric expansion is 3. Thermal expansion must be taken into account when welding, forging and hot stamping, manufacturing casting molds, dies, rolling rolls, gauges, making precision connections and assembling instruments, laying railway rails.
Heat capacity is the ability of a metal to absorb a certain amount of heat when heated. In SI units it has the dimension J/K. The heat capacity of various metals is compared by the specific heat capacity - the amount of heat, expressed in large calories, that is required to increase the temperature of 1 kg of metal by 1 ° C (in SI units - J / (kg K).
The ability of metals to conduct electric current is assessed by two mutually opposite characteristics - electrical conductivity and electrical resistance . Electrical conductivity is estimated in SI in siemens (S), and electrical conductivity - in S/m; similarly, electrical resistance is expressed in ohms (Ohm), and electrical resistivity - in Ohm m. Good electrical conductivity is necessary, for example, for current-carrying wires (copper, aluminum). In the manufacture of electric heating devices and furnaces, alloys with high electrical resistance (from nichrome, constantan, manganin) are required. As the temperature of a metal increases, its electrical conductivity decreases, and as it decreases, it increases.
Magnetic properties are characterized by absolute magnetic permeability or magnetic constant, i.e. the ability of metals to be magnetized. In SI units, the magnetic constant has the dimension Gn/m. Iron, nickel, cobalt and their alloys, called ferromagnetic, have high magnetic properties. Materials with magnetic properties are used in electrical equipment and for the manufacture of magnets.
Chemical properties characterize the ability of metals and alloys to resist oxidation or combine with various substances: atmospheric oxygen, acid solutions, alkali solutions, etc.
The easier a metal combines with other elements, the faster it breaks down. The chemical destruction of metals under the influence of an external aggressive environment on their surface is called corrosion . Metals that are resistant to oxidation under high heat are called heat-resistant or scale-resistant . Such metals are used for the manufacture of parts that are operated in high temperature zones.
The resistance of metals to corrosion, scale formation and dissolution is determined by the change in the mass of the tested samples per unit surface per unit time. The chemical properties of metals are necessarily taken into account in the manufacture of products. This especially applies to products or parts operating in chemically aggressive environments:
- containers for transporting chemical reagents;
- chemical pipelines;
- devices and instruments in the chemical industry, etc.
Chemical properties
Metals are reducing agents and react with non-metals to form oxides, hydroxides, and salts. The most active are the alkali and alkaline earth metals, located in groups I and II of the periodic table. Noble metals (Au, Ag, Pt) are low-active and do not interact with oxygen and water.
Rice. 2. Metal activity scale.
Features of the chemical interaction of metals with other elements are described in the table.
| Interaction | Products | The equation |
| With oxygen | Oxides | 2Mg + O2 → 2MgO |
| With sulfur | Sulfides | Zn + S → ZnS |
| With nitrogen | Nitrides | 6Li + N2 → 2Li3N |
| With phosphorus | Phosphides | 3Ca + 2P → Ca3P2 |
| With halogens | Halides | 2Na + Cl2 → 2NaCl |
| With water | Hydroxides | 2Na + 2H2O → 2NaOH + H2 |
| With acids | Salts | 2Al + 3H2SO4 → Al2(SO4)3 + 3H2 |
| With salts (replace less active metals) | Salt | 2Fe + Cu2SO4 → Fe2SO4 + 2Cu |
Gold dissolves in aqua regia (a mixture of hydrochloric and nitric acids), silver - in concentrated nitric and hot sulfuric acids.
Rice. 3. Gold.
Basic mechanical properties of metals
The ability of a metal to resist external forces is characterized by mechanical properties : strength, elasticity, ductility, toughness, hardness and endurance. These properties are determined by the results of mechanical tests, when metals are exposed to external forces (loads). External forces can be static, dynamic or cyclic (repeatedly variable).
Load causes stress and deformation in a solid. Stress is the magnitude of the load per unit cross-sectional area of the test sample. Deformation is a change in the shape and size of a solid body under the influence of applied external forces. There are tensile (compressive), bending, torsion, and shear deformations . A material can be subject to one or more types of deformation simultaneously.
To determine strength, elasticity and ductility, metals in the form of round or flat samples are tested for static tension (GOST 1497–84). Tests are carried out on tensile testing machines . As a result of the tests, a tension diagram is obtained. The abscissa axis of this diagram shows the strain values, and the ordinate axis shows the loads applied to the sample.
Strength is the ability of a material to resist destruction under loads; assessed by tensile strength and yield strength. An important indicator of the strength of a material is also the specific strength - the ratio of the tensile strength of the material to its density. Ultimate strength v (temporary resistance) is the conditional stress in Pa (N/m2), corresponding to the greatest load preceding the failure of the sample:
where Pmax is the greatest load, N; F0 is the initial cross-sectional area of the working part of the sample, m2.
True tensile strength Sk is the stress determined by the ratio of the load Pk at the moment of rupture to the area of the minimum cross-section of the sample after rupture:
The yield strength (physical) σt is the lowest stress (in megapascals) at which the sample is deformed without a noticeable increase in load:
where Pt is the load at which the yield plateau is observed, N. Basically, only low-carbon steel and brass have a yield plateau. Other alloys do not have yield plateaus.
Composition, structure and properties of metals
In the solid state, all metals and metal alloys have a crystalline structure with a strictly defined arrangement of atoms that form a regular crystal lattice. This ordered arrangement of atoms distinguishes crystalline materials from amorphous materials (glass), in which the atoms are arranged randomly. The number of atoms in different sections of the crystal lattice is not the same, therefore the mechanical, electrical and other properties of metals in different directions are different. This phenomenon is called anisotropy, and materials are called anisotropic.
Metals used in technology consist of a large number of crystals of regular and irregular shapes, which are called grains or crystallites. 1 cm3 of metal products (for example, rolled steel) contains tens of thousands of crystallites. Along the boundaries between metal grains, the correct structure of the crystal lattice is disrupted. In addition, even chemically pure metal contains impurities of foreign atoms that distort the crystal lattice. All these structural disturbances lead, first of all, to a significant decrease in actual strength. For example, the theoretical strength of iron is 1400 MPa, while the practical strength does not exceed 300 MPa.
Metals can, when heated, leading to destruction of the crystal lattice, transform into a viscoplastic state, and when the melt is cooled, into a crystalline state. This transition occurs at a strictly defined temperature, which is called the melting or crystallization temperature. Some metals (iron, tin, etc.) are capable of changing the shape and arrangement of crystals in the solid state with increasing temperature. The existence of the same metal in several crystalline forms with different arrangements of atoms in the lattice is called allotropy. A number of metals are capable of forming alloys - compounds of complex composition formed as a result of the interaction of two or more metals or metals with some non-metals. In construction, alloys of copper and aluminum, as well as cast iron and steel, which are compounds of iron and carbon, are most widely used.
The properties of metals and alloys depend on their composition and microstructure. This dependence, which is widely used in practice, was first established by academician N.S. Kurnakov (1880 – 1941). As a rule, the higher the melting point of a metal or alloy, the greater its strength and better thermal and electrical conductivity. To obtain alloys with specified properties, as well as to assess the reliability of metal structures, macroscopic and microscopic analyzes are used: macroscopic analysis is carried out with the naked eye or a magnifying glass is used with a magnification of up to 30 times on specially prepared samples; microscopic analysis consists of studying the structure and composition of metals and alloys using special optical and electron microscopes, where magnification can reach 3000 times or more.
The mechanical properties of metals depend on the type of load, the conditions of its action, and the ambient temperature. Strength characteristics are determined by testing standard samples or the products themselves on special machines. The test mode can be static - the load on the sample increases gradually (determining compressive, bending, tensile strength), dynamic - the load on the sample acts instantly (impact test), and repeatedly variable - the load on the sample changes many times in magnitude and direction ( fatigue test). Metals are tested for tension, compression, torsion, impact, fatigue, hardness, and creep at room, low and high temperatures.
Tensile testing is carried out using tensile testing machines. Based on the magnitude of the tensile loads and the corresponding elongations of the sample, a tensile diagram is drawn that characterizes the behavior of the metal or alloy under load until failure. impact testing, a pendulum pile driver is used, which allows one to determine the ability of a metal to withstand dynamic loads and identify its tendency to brittle fracture at different temperatures. Fatigue testing evaluates the ability of metals (alloys) to work under the action of repeated loads varying in magnitude and sign. The ability of metals to withstand a large number of test cycles is called endurance. Tests are carried out on cylindrical samples by subjecting them to rotating bending loads, which cause alternating stresses and bring the sample to destruction.
To determine hardness in practice, various methods are used based on the introduction into a metal surface of a tip made of a particularly hard material (hardened steel, diamond, sapphire) and having the correct shape in the form of a ball, cone or pyramid. The most widely used method is the Brinell method, which is based on calculating the hardness based on the diameter of the imprint of a metal ball of a certain mass and diameter pressed into the surface. Test conditions limit the magnitude and duration of the applied load.
When studying the properties of metals (alloys), much attention is paid to the study of their destruction processes under the action of gaseous and liquid media under conditions of normal and high temperatures. The importance of this work is emphasized by the fact that annually 30% of the metal produced is used to restore losses from corrosion, of which 10% is lost irretrievably.
Corrosion starts from the surface of the metal and spreads deeper. The intensity of corrosion destruction depends mainly on three factors: the first is the chemical composition and microstructure of the metal or alloy; the second is the chemical composition of the environment and the percentage of aggressive substances in it (oxygen, acids, alkalis); the third is the ambient temperature. Depending on the reasons causing the destruction, corrosion can be chemical and electrochemical.
Surface destruction of metal under the influence of gases at high temperatures or organic liquids (alcohol, gasoline, oil, fuel oil, etc.) is called chemical corrosion. An example of chemical corrosion is the oxidation process at high temperatures of metal fittings of furnaces, valves of internal combustion engines, and gas turbine blades.
Electrochemical corrosion of metal products occurs in various aqueous solutions that conduct electric current. This is the most common type of corrosion. It is observed in atmospheric conditions, at sea, in soil, groundwater, and in solutions of various acids and salts. A significant part of building metal structures and products (frames and roofs of buildings, bridge trusses, reinforcement in reinforced concrete) is subject to electrochemical corrosion. The essence of the electrochemical corrosion process is that the atoms located in the nodes of the crystal lattice, upon contact with the electrolyte, go into solution in the form of ions, causing the destruction of the metal.
There are several types of corrosion damage: uniform, occurring over the entire surface at the same speed; uneven - continuous, the speed of which in certain areas depends on the structure of the alloy and the presence of defects on the surface of the products; local or local, observed on individual areas of the surface of the metal (alloy).
One of the ways to prevent corrosion is to eliminate the conditions that cause it: heterogeneity of structure, the presence of defects on the surface of products, uneven illumination and thermal heating. protection methods are used to combat corrosion : the introduction of alloying additives into the composition, electrochemical protection (anodic or cathodic), treatment of the corrosive environment and protective coatings. The protective effects of alloying additives – Cu, Al, Ti, V, Cr, Ni, Co, etc., which are introduced to change the structure and properties of metals, are caused either by the formation of corrosion-resistant oxide films on the surface of products, or by the creation of alloys that are highly resistant to aggressive Wednesdays
metal coatings are increasingly used which are applied by galvanic and hot methods, metallization, and cladding. With the galvanic method, a thin protective layer of some metal is created on the surface of the product by electrolytic deposition from a salt solution. An example is the galvanizing of embedded parts for reinforced concrete structures. With the hot method, products are immersed in a bath of molten protective metal (zinc, tin, lead). Metallization is a common method of protection in construction. It consists of applying compressed air a thin layer of sprayed molten metal (zinc, aluminum) onto the surface of a metal product or structure to be protected from corrosion. Another option for protecting metal coatings from corrosion is cladding.
Cladding is a thermomechanical method for producing two- and multilayer metals (bimetals), firmly connected to each other along the entire contact plane. Metallurgical plants organize the production of sheets, wire, pipes coated with zinc, aluminum, and silicon.
Protection against corrosion of load-bearing and enclosing metal structures in the conditions of a construction and installation site is carried out with paint and varnish compositions based on bitumen, polymers and other materials. This direction is a priority at present, since at the lowest energy costs it is possible to obtain a reliable, durable coating. The compositions used include zinc-containing, thermosetting paints based on high-molecular resins (epoxy, polyester). The latter are applied to the surface of metal products and structures in an electric field using a spray gun with a high voltage source located inside. Their advantage lies in the absence of toxic solvents, the possibility of using waste-free application technology, the absence of a primer layer and the high quality of the fused, dense coating, which has increased impact strength, corrosion resistance, and adhesion to the protected surface. Depending on the purpose, it can be thin-layered and textured, with a thickness ranging from less than 1 micron to 20 microns or more. The optimal protection option is chosen depending on the material of construction, the degree of aggressive impact on it and technical and economic feasibility. If it is necessary to ensure particularly reliable and durable protection of steel structures, combined coatings are used, for example, metal and paint.
The destructive effect of a corrosive environment on metals and alloys can also be reduced by introducing special additives into its composition - inhibitors that slow down the corrosion process.
Destructive factors also include the effect of fire on metal products and structures . Under the influence of an open flame and high temperature, metals soften, deform and crack.
Unprotected steel structures, depending on the thickness of the elements, cross-section and operating stresses, have a fire resistance limit of 0.1 - 0.44 hours. When exposed to fire, the load-bearing capacity of metal structures decreases due to a decrease in the strength and elasticity of the metal when heated, as well as due to the appearance of plastic and temperature deformations. Metals are fireproof materials, but have high thermal conductivity, so their fire protection consists of creating heat-insulating screens on the surface of metal structural elements that provide high resistance to fire and high temperatures. Traditional means of fire protection for metal structures are heavy and light concrete, brick, and cement-sand plasters. These materials can create almost any fire resistance limit for structures. So, to ensure a fire resistance limit of a steel structure of 2 hours, a layer of heavy concrete or gypsum 60 mm thick, plaster - 50 - 60 mm, brick - 65 mm are required.
In recent years, heat-insulating plasters and fire-retardant coatings based on clay, liquid glass, gypsum with the use of expanded perlite, vermiculite, asbestos and mineral kaolin fiber, which have high thermal insulation properties, as fire-resistant aggregates and fillers have been increasingly used. The compositions are applied to the surface of metal structures with a spray gun. Depending on the required fire protection (45 - 150 min) and the thickness of the metal in the structure, the thickness of the protective layer ranges from 8 to 40 mm.
One of the promising means of fire protection is intumescent paints, consisting, for example, of a solvent, an acrylic polymer and a foaming fire retardant, which are applied to the surface of metal structures in a thin layer (1 - 1.2 mm). At a temperature of about 170 °C, the paint swells, forming a porous thermal insulation layer, the thickness of which is several centimeters. Due to its low thermal conductivity, the foam mass prevents rapid heating of the metal, increasing fire resistance up to 1 hour. In addition, for fire protection of metal structures, slab and sheet heat-insulating materials in the form of asbestos-cement and asbestos-gypsum facing boards, plasterboard and gypsum-fiber sheets.
As operating practice has shown, the cause of destruction of metal structures can also be the accumulation on their surface of waste products of microorganisms: organic acids, sulfides, hydrogen sulfide, ammonia - biological corrosion. To protect metal structures from biological damage mastic and paint are used with the introduction of effective biocidal additives.
Elasticity, plasticity, viscosity
Elasticity is the ability of a material to restore its original shape and dimensions after the cessation of the load Rup, it is assessed by the limit of proportionality σпз and the elastic limit σуп. Limit of proportionality σпс - stress (MPa), above which the proportionality between the applied stress and the deformation of the sample is violated:
where F0 is the initial cross-sectional area of the working part of the sample, m2; Rpc - proportional limit load, N.
Elastic limit (conditional) σ0.05 is the conditional stress in megapascals corresponding to the load at which the residual deformation first reaches 0.05% of the design length of the sample l0:
where P0.05 is the elastic limit load, N.
Plasticity , i.e. the ability of a material to take on a new shape and size under the influence of external forces without collapsing is characterized by relative elongation and relative contraction. Relative elongation (after rupture) σ is the ratio of the increment (lк – l0) of the estimated length of the sample after rupture to its initial estimated length l0, expressed as a percentage: δ = [(lк – l0) / l0] · 100%. Relative narrowing (after rupture) ψ is the ratio of the difference between the initial and minimum areas (F0 – Fк) of the cross-section of the sample after rupture to the initial cross-sectional area F0, expressed as a percentage: Ψ = [(F0 – Fк)/F0] 100% . The greater the relative elongation and contraction values for a material, the more ductile it is. For brittle materials these values are close to zero. The fragility of a structural material is a negative property. To eliminate this property, the metal is alloyed or heat treated.
Impact strength , i.e. the ability of a material to resist dynamic loads is defined as the ratio of the work W (in MJ) spent on breaking a sample to its cross-sectional area F (in m2) at the incision site KS = W/F. For testing (GOST 9454–78), special standard samples are made in the form of square blocks with a notch. The sample is tested on pendulum pile drivers. Determination of impact strength is important for some metals that operate at sub-zero temperatures and exhibit a tendency to cold brittleness.
Cyclic viscosity is the ability of materials to absorb energy under repeated varying loads. Materials with high cyclic toughness quickly dampen vibrations, which are often the cause of premature failure.
Hardness, fatigue, endurance
Hardness is the ability of a material to resist the penetration of another, harder body into it. Metal-cutting tools must have high hardness: cutters, drills, cutters, as well as surface-hardened parts. The hardness of the metal is determined by the Brinell , Rockwell and Vickers .
The measure of the hardness of the NV is taken as the ratio of the load to the surface area of the print with a diameter d and depth t, which is formed when a ball with a diameter D is pressed by force P. The numerical value of hardness is determined as follows: the diameter of the print is measured using an optical magnifying glass (with divisions), and from the resulting value is found in table attached to GOST, the corresponding hardness number. To assess the hardness of metals in small volumes, for example, on metal grains or its structural components, a method is used to determine microhardness .
Fatigue is the process of gradual accumulation of damage to a material under the influence of repeated alternating stresses, leading to the formation of cracks and destruction. Metal fatigue is caused by the concentration of stress in its individual volumes, in which there are non-metallic inclusions : gas bubbles, various local defects, etc. Characteristic is a fatigue fracture , which forms after the destruction of a sample as a result of repeated loading and consists of two parts that are different in appearance. One part of the fracture with a smooth (worn) surface is formed due to the friction of surfaces in the area of cracks arising from the action of repeatedly variable loads, the other part with a granular fracture occurs at the moment of destruction of the sample. Fatigue tests are carried out
on special machines. The most common machines are for repeated-alternating bending of a rotating sample, fixed at one or both ends, as well as machines for testing tension-compression and repeated-alternating torsion. As a result of the tests, the endurance limit is determined, which characterizes fatigue resistance.
Endurance is the property of a material to resist fatigue. The endurance limit is the maximum stress that a metal can withstand without failure for a specified number of loading cycles. There is an approximate relationship between the endurance limit and the strength limit
where σ-1 and σ-1р are the endurance limits for bending and tension-compression, respectively; σв is the tensile strength.
Tests for impact strength, fatigue strength, creep
In addition to static tests for impact strength , products are tested under the influence of impact (dynamic) alternating loads and high temperatures. For testing, a standard notched sample is used, which is installed on the supports of the pile driver. A pendulum of a certain mass is raised to a set height H, it is secured, and then the pendulum, released from the latch, falls, destroys the sample and rises again to a certain height h. This method of determining impact strength is the simplest. To facilitate calculations, use tables in which the work of impact is indicated for each rise of the pendulum after the destruction of the sample.
Fatigue strength tests . Car axles, crankshafts, turbine blades, leaf springs, springs, etc. are susceptible to fatigue. Careful grinding and polishing
and hardening the surface of parts significantly increase fatigue resistance and increase the service life of the product. Fatigue strength tests are carried out on various machines depending on the nature of the parts. The most common machines for testing are: 1) rotary bending; 2) during tension-compression; 3) during torsion.
Creep tests . Many machine parts operate under static loads at elevated temperatures. These are parts of steam and gas turbines, chemical, oil equipment, etc.
Creep is the property of a metal to slowly and continuously elongate (“crawl”) under the influence of constant operating stresses applied to it under conditions of elevated and high temperatures. While lead, aluminum and many alloys exhibit creep already at a temperature of 20°C, steel exhibits noticeable creep only starting at a temperature of 350–400°C. A quantitative characteristic of creep is the so-called creep limit . Creep tests are carried out in special installations that contain heating devices and instruments. Low-temperature installations are also used, which primarily affect the strength and ductility of products.
Technological and operational properties
Technological properties characterize the ability of metals to be processed in cold and hot states. The main technological properties include machinability, weldability, malleability, casting properties, etc.
Cutting machinability is one of the most important technological properties, because the vast majority
blanks, as well as parts of welded assemblies and structures are subjected to mechanical processing. Some metals are processed well, to obtain a clean and smooth surface, while others, which have high hardness, are processed poorly. The workability of steel, for example, can be improved by heat treatment, reducing or increasing the hardness of the material.
Weldability is the ability of metals to form a welded joint whose properties are close to those of the base metal. It is determined by bending or tensile testing of the welded sample.
Malleability is the ability of a metal to be processed by pressure in a cold or hot state without signs of destruction. It is determined by a forging test for upsetting to a given degree of deformation. If a crack does not form on the side surface of the sample, then such a sample is considered to have passed the test, and the tested metal is considered suitable for pressure treatment.
The casting properties of metals characterize their ability to form castings without cracks, cavities and other defects. Fluidity is the ability of molten metal to fill the cavity of a casting mold well.
Shrinkage during crystallization is a decrease in the volume of a metal during the transition from a liquid to a solid state; causes the formation of shrinkage cavities and shrinkage porosity in ingots and castings. Liquation is the heterogeneity of the chemical composition of alloys that occurs during their crystallization. Liquation is due to the fact that alloys, unlike pure metals, crystallize not at a fixed temperature, but in a temperature range.
Operational properties are determined depending on the operating conditions of the machine by special tests. Wear resistance is the property of a material to resist wear, i.e. a gradual change in the size and shape of the body due to the destruction of the surface layer of the product due to friction. Performance properties also include cold resistance, heat resistance , anti-friction , etc.