Concept of metallurgy
Metallurgy - the extraction of metals from ores - is one of the oldest types of human activity. Back in the second millennium BC. e. in Egypt they knew how to smelt iron from iron ore. The so-called Iron Age replaced the Bronze Age, which, in turn, came after the Stone Age.
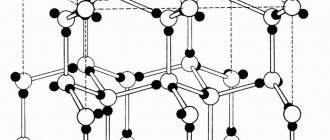
Metals are obtained from ore minerals. For example, chalcopyrite or copper pyrite is a raw material for the production of iron, copper and sulfur ( Fig. 1 ). The chemical formula of the mineral is CuFeS2. Metals in other ores are in the form of oxides or salts of inorganic acids, chemically bonded cations.
Rice. 1. Chalcopyrite
The essence of the metallurgical process is the reduction of positive ions to free metal atoms. Carbon and its compounds, hydrogen, and metals are used as sources of electrons. During the reduction process, cations receive the missing electrons. The electron shells of the metal are restored. Process diagram:
Ме+n + ne- → Me, where
- Ме+n—metal in oxidized form;
- +n—oxidation state;
- ne- is the number of added electrons;
- Me is a metal in reduced form.
Improving the properties of alloys
By fusing certain metals and other chemical elements, materials with improved characteristics can be obtained. For example, the yield strength of pure aluminum is 35 MPa. When producing an alloy of this metal with copper (1.6%), zinc (5.6%), magnesium (2.5%), this figure exceeds 500 MPa.
By combining different chemicals in different proportions, metallic materials with improved magnetic, thermal or electrical properties can be obtained. The main role in this process is played by the structure of the alloy, which is the distribution of its crystals and the type of bonds between atoms.
Methods for obtaining metals
Depending on what reducing agent is used in the metallurgical process, they distinguish: pyro-, hydro, electro- and biometallurgy.
The most common methods for producing metals are pyrometallurgical and electrometallurgical. Most reduction reactions occur at high temperatures ( Fig. 2 ). Since the metallic bond has increased strength, the isolation of pure metals from natural compounds is carried out at high temperatures.
Rice. 2. Metallurgical production
Pyrometallurgical method
Pyrometallurgy is the extraction of metals from ores at high temperatures with the participation of reducing agents. Translated from Greek, “pyros” means “fiery.” Coke, carbon dioxide, and hydrogen are used as reducing agents. Active metals are used to obtain less active ones.
Pyrometallurgy is divided into
- carbothermy,
- hydrogenothermy,
- metallothermy.
Carbothermy: conversion of metal sulfide by firing into oxide and further reduction with coal to a pure state.
2ZnS + 3O2 = 2ZnO + 2 SO2
ZnO + C = CO + Zn
Ores consisting of iron oxides and sulfides are subjected to carbothermy. Reduction is carried out with coke or carbon dioxide (carbon monoxide). Iron alloys are produced - cast iron and steel. The first contains more carbon, as well as oxides of sulfur, phosphorus and silicon. Carbon reduces hardness and other qualities characteristic of metals.
Chemical reactions underlying iron smelting:
- C + O2 = CO2↑,
- CO2 + C ↔ 2CO↑,
- 3Fe2O3 + CO = 2Fe3O4+ CO2↑,
- Fe3O4 + CO = 3FeO + CO2↑,
- FeO + CO = Fe + CO2↑.
Steel is smelted in special furnaces - electric, converter, open-hearth ( Fig. 3 ). When blowing oxygen-enriched air, excess carbon burns out, its content decreases to 2% and below. This method is more economically applicable, because it is used to produce steel and cast iron, which are widely used in modern industry.
Rice. 3. Pyrometallurgy
By reducing coal you can obtain iron, copper, zinc, cadmium, germanium, tin, lead and other metals. Copper (Cu2O), tin (SnO2), manganese (MnO2) ores are used as raw materials.
| Scheme for obtaining iron and chromium | (Cr2Fe)O4 + 4C(coke) = Fe + 2Cr + 4CO↑ |
| The reaction underlying copper smelting | Cu2O + C (coke) = 2Cu + CO↑ |
| Tin production diagram | SnO2 + 2C (coke) = Sn + 2CO↑ |
| Manganese smelting process | MnO2 + C(coke) = Mn + CO2↑ |
| Lead production scheme | 2PbO + C → Pb + CO↑ |
Metals can be extracted from sulfide ores. First, firing is carried out, then the resulting oxide is reduced with coal. Schemes for roasting zinc blende and obtaining zinc:
- 2ZnS + 3O2 = 2ZnO + 2SO2↑;
- ZnO + C = Zn + CO↑.
Carbonates are also calcined with coal to produce oxides and subsequent reduction with coal. Schemes for firing siderite and reducing iron oxide:
- FeCO3 = FeO + CO2↑;
- FeO + C = Fe + CO↑.
Hydrogenothermy - production of metals by reduction with hydrogen
The advantage of this metallurgical method is the production of very pure metals. The reduction of copper from CuO oxide is an example of the reducing properties of hydrogen from a school course in inorganic chemistry. Reaction flow diagram (Fig. 4 ):
Rice. 4. Reduction of copper with hydrogen
Refractory metals molybdenum and tungsten are reduced from oxides with hydrogen.
Metallothermy
One metal is reduced by another, more chemically active one. This method is used to obtain metals from oxides and halides.
Depending on the nature of the reducing metal, aluminothermy or aluminothermy - reduction with aluminum and magnesiumthermy - reduction with magnesium are distinguished.
| Manganese production scheme | 3MnO2 + 4Al = 3Mn + 2Al2O3 |
| Chromium smelting process | Cr2O3 + 2Al → 2Cr + Al2O3 |
| Scheme for obtaining calcium | 4CaO+ 2Al= 2Ca+ (CaAl2)O4 |
Siliconothermy is the reduction of metals with silicon. The process proceeds according to the scheme: 2MgO + Si → 2Mg + SiO2.
Pyrometallurgy
Pyrometallurgy
is a set of metallurgical processes occurring at high temperatures. This is a branch of metallurgy associated with the production and purification of metals and metal alloys at high temperatures, in contrast to hydrometallurgy, which involves low-temperature processes.
Description[ | ]
These are chemical processes occurring in metallurgical units at high (800-2000°C) temperatures. Therefore, pyrometallurgy is sometimes called “high temperature chemistry.”
Often chemical reactions are accompanied by a change in the aggregate state of the reacting substances: melting, sublimation, evaporation of the resulting metals or their compounds.
In such processes, interactions can occur between solid, liquid (melts) and gaseous phases in any combination.
Pyrometallurgical processes are the processes of agglomeration of metallurgical raw materials, smelting of charge materials, production of alloys, and refining of metals. In particular, this is roasting, blast furnace smelting, smelting in converters, arc and induction furnaces. Pyrometallurgy is the basis for the production of cast iron, steel, lead, copper, zinc, etc.
In pyrometallurgy, carbon reduction is often used - in cases where the metals being reduced do not form stable carbides, in addition to those mentioned above, such metals include germanium, cadmium, tin and others. In cases where stable carbides are formed by reduced metals, metallothermy is often used instead of reduction with carbon[1].
Pyrometallurgy is the main and most ancient field of metallurgy. From ancient times until the end of the 19th century, metal production was based almost exclusively on pyrometallurgical processes.
At the turn of the 19th and 20th centuries, another major branch of metallurgy, hydrometallurgy, acquired industrial importance.
However, pyrometallurgy continues to maintain a dominant position both in terms of production scale and variety of processes.
At the beginning of the 20th century, along with flame heating methods, different types of electric heating (arc, induction, etc.) began to be used in metallurgy; Around the same time, electrolysis of molten chemical compounds was introduced into industry (production of aluminum and other non-ferrous metals).
In the 2nd half of the 20th century, plasma melting of metals, zone melting, etc. became widespread. Metallurgical processes based on the use of electric current are classified as an independent field of pyrometallurgy - electrometallurgy.
Basic processes[ | ]
The main process of pyrometallurgy is ore smelting, which is carried out at such high temperatures that the products of chemical interaction melt, forming two liquid phases - metal or sulfide and slag. There are reduction and oxidation melts.
The defining process of reduction smelting is the reduction of metal oxides to ultimately produce a molten metal or its alloy with other elements. A typical reduction smelting process is the production of pig iron in blast furnaces. Reduction processes are also the main ones in the smelting of manganese, oxidized nickel, lead, and titanium ores.
The main reducing agents are carbon, carbon monoxide and hydrogen. Carbon monoxide is formed in the furnace itself when carbon is not completely burned; The main amount of hydrogen is obtained as a result of the decomposition of natural gas blown into the furnace.
A type of reduction smelting is the metallothermic production of metals, in which another metal with a greater affinity for oxygen is used as a reducing agent for a certain metal (Mn, Cr, V, etc.): Ca; Mg; Al and also Si. One of the advantages of metallothermic reduction is the production of metals that are not contaminated by carbon or hydrogen.
A typical oxidative ore smelting process is the processing of rich copper sulfide ores in shaft furnaces. During smelting, the bulk of the sulfur in sulfide minerals is oxidized, resulting in the release of a significant amount of heat. The main target product of smelting is the melt of FeS and Cu2S sulfides - matte.
Pig iron and ore smelting matte are essentially intermediate products that require additional processing. This treatment involves blowing the melts with air or pure oxygen, as a result of which the impurities contained in the alloys are oxidized and pass either into slag (SiO2; MnO; FeO, etc.) or into gas (CO; SO2). The process is called conversion.
A similar process to conversion is the fumming process—blowing slag melts with gas. Its difference from converting is that the metal melt is blown with an oxidizing gas, and when fumigating the slag, with a reducing gas.
And secondly, the products of oxidation of the metal melt - metal oxides - form a second liquid phase - slag, and the products of slag fuming - reduced volatile metals (or sulfides) in a vapor state are removed from the reaction space by a gas flow [2].
Literature[ | ]
Hydrometallurgical method
Hydrometallurgy is a method for producing noble, non-ferrous and rare metals. For example, copper oxide is first converted to sulfate using sulfuric acid. Copper is displaced from solution by iron. The following substitution reaction occurs: CuSO4 + Fe = Cu + FeSO4. Or copper is removed from solution by electrolysis. An electric current is passed through, Cu2+ ions are deposited on the cathode.
The advantage of the hydrometallurgical method is the ability to obtain metals from low-grade ores. Another advantage of the method is the reduction of gaseous emissions into the atmosphere. Large amounts of harmful gases and soot are released into the air during ore roasting and pyrometallurgy.
Properties of alloys
Regardless of what methods of producing metals and alloys are used, their properties are completely determined by the crystalline structure of the phases and the microstructure of these materials. Each of them is different. The macroscopic properties of alloys depend on their microstructure. In any case, they differ from the characteristics of their phases, which depend solely on the crystal structure of the material. Macroscopic homogeneity of heterogeneous (multiphase) alloys is obtained as a result of the uniform distribution of phases in the metal matrix.
The most important property of alloys is weldability. Otherwise they are identical to metals. Thus, alloys have thermal and electrical conductivity, ductility and reflectivity (brilliance).
Part 2. Oxides, preparation and properties. Obtaining oxides:
| Methods of obtaining. | Examples. | Limitations and Notes |
| 1. Oxidation of simple substances: | a) metals: 2Ca + O2 2CaO b) non-metals: 4P + 3O2 (wk) 2P2O 3 4P + 5O2 (g) 2P2O5 (From S – SO2, from Fe – Fe2O3 and Fe3O4, from N2 – NO) | Halogens, inert gases, Au, Pt do not react with oxygen. Nitrogen reacts under harsh conditions (2000°C). |
| 2. Oxidation of complex substances: | a) hydrogen compounds: 2H2S + 3O 2 2H2O + 2SO 2 b) sulfides, carbides, phosphides (binary compounds): 2ZnS + 3O2 2ZnO + 2SO2 | Each element of a complex substance is oxidized in accordance with its properties. |
| 3. Decomposition of hydroxides and salts: | a) hydroxides (bases and acids): 2Al(OH)3→t Al2O3 + 3H2O H2SiO3 →t SiO2 + H2O b) carbonates: CaCO3→t CaO+CO2 | Alkali metal hydroxides and carbonates (Na, K, Rb, Cs) do not decompose. |
| 4. Oxidation with oxygen or ozone | a) oxygen: 2CO + O2 2CO2 b) ozone: NO + O3 NO2 + O2 | Possible if the element has several oxides (sulfur, phosphorus, carbon, nitrogen, iron). |
Properties of oxides.
Basic oxides
– oxides to which bases correspond. These are metal oxides with oxidation states +1 and +2, except for amphoteric ones (ZnO, BeO, SnO, PbO)
Properties of basic oxides.
| Properties | Examples of reactions | Limitations and Notes |
| 1) Reaction with acid solutions | Li2O + 2HCl= 2LiCl+ H2O NiO + H2SO4 = NiSO4 + H2O | The acid must exist in the form of a solution (silicic, hydrogen sulfide, and carbonic acids do not react) |
| 2) Reaction with water | Li2O + H2O = 2LiOH BaO + H2O = Ba(OH)2 (only 8 oxides: group IA, CaO, SrO, BaO) | An oxide reacts with water only if it results in soluble hydroxide (alkali). |
| 3) Reaction with acidic and amphoteric oxides | BaO + CO2 = BaCO3, FeO + SO3 = FeSO4, CuO + N2O5 = Cu(NO3) 2 CaO + SO2 = CaSO3 | One of the reacting oxides (basic or acidic) must correspond to a strong hydroxide. |
| 4) Reduction of oxide to metal or to lower oxide : | MnO + C = Mn + CO (when heated), FeO + H2 = Fe + H2O (when heated). Fe2O3 + CO = FeO + CO2 | As reducing agents use: CO, C, hydrogen, aluminum, magnesium. Oxides of inactive metals react with hydrogen. |
| 5) Oxidation with oxygen. | 4FeO + O2 = 2Fe2O3 | If a metal has several oxides with different oxidation states. |
Acidic oxides
– oxides, which correspond to acids.
Acidic oxides at room temperature are:
*gases
(for example: CO2, SO2, NO, SeO2)
*liquids
(for example, SO3, Mn2O7)
*
solids (for example: B2O3, SiO2, N2O5, P2O3, P2O5, I2O5, CrO3).
Properties of acid oxides.
| Properties | Examples of reactions | Notes |
| 1 ) Reaction with bases | CO2 + Ca(OH) 2 = CaCO3 + H2O SiO2 + 2KOH = K2SiO3 + H2O (when heated), SO3 + 2NaOH = Na2SO4 + H2O, N2O5 + 2KOH = 2KNO3 + H2O. | The reaction is possible with alkalis. The most active acid oxides (SO3, CrO3, N2O5, Cl2O7) can also react with insoluble (weak) bases. |
| 2) Reaction with amphoteric and basic oxides | CO2 + CaO = CaCO3 P2O5 + 6FeO = 2Fe3(PO4)2 (when heated) N2O5 + ZnO = Zn(NO3)2 | One of the reacting oxides (basic or acidic) must match strong hydroxide . |
| 3) Reaction with water. are formed . | N2O3 + H2O = 2HNO2 SO2 + H2O = H2SO3 N2O5 + H2O = 2HNO3 SO3 + H2O = H2SO4 | An oxide reacts with water if it results in soluble hydroxide |
| 4) Reactions with salts of volatile acids. | SiO2 + K2CO3 = K2SiO3 + CO2 (when heated) | Solid, non-volatile oxides (SiO2, P2O5) displace volatile ones from salts. |
| 5) Oxidation . | 2SO2 + O2 ⇆ 2SO3 | Lower oxides are oxidized to higher ones. |
Amphoteric oxides
– oxides that can react with both acids and alkalis.
In terms of their chemical properties, amphoteric oxides are similar to basic oxides and differ from them only in their ability to react with alkalis
, both solid (when fused) and with solutions,
as well as with basic oxides.
Substances formed by amphoteric metal cations in an alkaline medium:
| Oxidation state | In solution | In the melt |
| +2 ( Zn , | Na 2 [ Zn ( OH ) 4 ] sodium tetrahydroxozincate | Na2ZnO2 sodium zincate |
| +3 ( Al , | Na[Al(OH)4] sodium tetrahydroxyaluminate Na3 [ Al ( OH ) 6 ] _ sodium hexahydroxyaluminate | NaAlO2 sodium metaaluminate and Na 3 AlO 3 sodium orthoaluminate |
| *) iron does not form stable hydroxo complexes, it is amphoteric only in the melt, forming NaFeO 2 | ||
PROPERTIES OF AMPHOTERIC OXIDES.
| Properties | Examples of reactions | Notes |
| 1) Reacts with acids , just like basic oxides - salts are formed. | ZnO + 2HCl = ZnCl2 + H2O Al2O3 + 6HNO3 = 2Al(NO3)3 + 3H2O | Only with strong acids |
| 2) Interact with alkali solutions - solutions of hydroxo complexes are formed. | Al2O3 + 2KOH +3H2O = 2K[Al(OH)4] or K3[Al(OH)6] ZnO +2NaOH +H2O=Na2[Zn(OH)4] | |
| 3) React with alkali melts - forming salts, while exhibiting the properties of acidic oxides. | Al2O3 + 2KOH →t 2KAlO2 + H2O (or K3AlO3) ZnO + 2KOH → t K2ZnO2 + H2O | |
| 4) When fused, they can interact with alkali metal carbonates , as with alkalis. | Al2O3 + Na2CO3 → t 2NaAlO2+CO2 (or Na3AlO3) ZnO + Na2CO3 → t Na2ZnO2+ CO2 |
studfiles.net
Steels and cast irons
These alloys are obtained by combining iron and carbon (2%). When producing alloyed materials, nickel, chromium, and vanadium are added to them. All ordinary steels are divided into types:
• low-carbon (0.25% carbon) is used for the manufacture of various structures;
• high-carbon (more than 0.55%) is intended for the production of cutting tools.
Various grades of alloy steels are used in mechanical engineering and other products.
An alloy of iron and carbon, the percentage of which is 2-4%, is called cast iron. This material also contains silicon. Various products with good mechanical properties are cast from cast iron.