Electrical and thermal conductivity of metals - Chemist's Handbook 21
Metals are formed from atoms of electropositive elements. In alloys, certain sites in the lattice can be occupied either by atoms of a single component or by different types of atoms. The high electrical and thermal conductivity of metals is due to the movement of free electrons through the spatial lattice. [p.583] Electrical and thermal conductivity of metals [p.218]
Electrical and thermal conductivity of metals are explained by the mobility of electrons in incompletely filled zones, due to the fact that in these zones the levels occupied by electrons are closely adjacent to free levels to which they can move (excite) electrons. [p.91]
The assumption that electrons in a metal move freely even in the absence of an electric field is confirmed by a number of experimental facts. Thus, a universal connection is discovered between the electrical conductivity and thermal conductivity of metals. The thermal conductivity of metals is much higher than the thermal conductivity of insulators; it has been found that the ratio of electrical conductivity and thermal conductivity, at least at average temperatures, is a universal function of temperature and does not depend on the nature of the metal (Wiedemann-Franz law). This indicates the commonality of the mechanism of both processes: heat transfer, like the transfer of electricity, is carried out due to the movement of free electrons; therefore, there are free electrons in the metal even in the absence of an electric field. The fact of the existence of freely moving electrons in metals is also confirmed by the phenomenon of thermionic emission (the emission of electrons by heated metals). It should be noted that the distribution of electron velocities in a metal, as experience shows, is Maxwellian. Thus, the presence of electron gas in metals can be considered experimentally confirmed. Assuming that the electron gas in a metal has the properties of a classical ideal gas, Drude gave a theoretical interpretation of the experimentally observed relationship between thermal conductivity and electrical conductivity. A number of thermoelectric phenomena have been explained. True, discrepancies have arisen between the theoretical and experimental values of the heat capacity of metals. According to the classical law of equidistribution of energy, the electron gas should contribute to the heat capacity of the metal equal to 3/2 R a 1 mole of free electrons (if the metal is monovalent, this is a contribution per 1 mole of substance). However, it has been experimentally established that the contribution of electrons to the heat capacity is practically zero. This contradiction found an explanation based on [p.183]
Table
2 also shows that the electrical conductivity and thermal conductivity of metals do not change too much (no more than 2.5 times) during melting. Similar results were obtained [9] for Fe, Co and Ni, for which the Jat/liz ratios are 1.07, 1.11 and 1.14, respectively. The magnetic susceptibilities of N1 and Co change little during melting (9]. [p.14]
The extremely high values of electrical and thermal conductivity of metals compared to other types of crystals indicate high mobility and greater freedom of electrons in their spatial structure. From the point of view of the structure of atoms typical [p.79]
In a metal, the number of atomic orbitals involved in the formation of an individual molecular orbital is extremely large, since each atomic orbital overlaps with several others. Therefore, the number of emerging molecular orbitals also turns out to be very large. In Fig. Figure 22.20 schematically shows what happens as the number of atomic orbitals increases, the overlap of which creates molecular orbitals. The energy difference between the highest and lowest energy molecular orbitals does not exceed the value characteristic of an ordinary covalent bond, but the number of molecular orbitals with energies falling in this range turns out to be very large. Thus, the interaction of all valence orbitals of metal atoms with the valence orbitals of neighboring atoms leads to the formation of a huge number of molecular orbitals extremely close to each other in energy, delocalized throughout the metal crystal lattice. The differences in energy between individual orbitals of metal atoms are so small that for all practical purposes the corresponding energy levels can be considered to form a continuous band of allowed energy states, as shown in Fig. 22.20. The valence electrons of the metal do not completely fill this band. You can simply imagine the energy zone of a metal as a vessel partially filled with electrons. This incomplete population of allowed energy levels by electrons is precisely what determines the characteristic properties of metals. Electrons occupying the orbitals of the highest occupied levels require very little excess energy to become excited and move to the orbitals of higher unoccupied levels. In the presence of any source of excitation, such as an external electric field or an influx of thermal energy, electrons are excited and move to previously unoccupied energy levels and thus can move freely throughout the crystal lattice, which determines the high electrical and thermal conductivity of the metal. [p.361]
Metals are characterized by a specific luster, high electrical conductivity, thermal conductivity and ductility. At the same time, metal vapors are the same dielectrics as inert gases, and differ from the latter in their relatively low ionization energy. The high electrical and thermal conductivity of metals and their thermionic emission are determined by the presence of free electrons. It is believed that when atoms come closer together during the formation of a metal, delocalization of valence electrons occurs. A metal is considered as a system of positive ions regularly located in space and delocalized electrons moving among them. These electrons compensate for the repulsive forces between the ions and bind them into a single crystal lattice.
What physical properties of metals are determined by the metallic bond?
Position of metals in the periodic table
The list of simple substances compiled by the great French chemist Lavoisier in 1789 contains 17 metals; in the first version of the periodic table D.I. Mendeleev (1869) – there are already 47 of them. Of the 114 chemical elements, 92 are metals. In the traditional version of the Periodic Table, the metal elements are located at the beginning of the periods, as well as in secondary subgroups. The conventional boundary separating metals from non-metals is the straight line drawn from boron to astatine in the long version of the periodic table. Metals are to the left and below this line, non-metals are to the right and above, and elements located near the line have a dual nature, sometimes called metalloids . In the Periodic Table approved by IUPAC, metals are located in groups 1-12.
STRUCTURE FEATURES OF METALS
Metal atoms at the outer level contain no more than four electrons, usually from one to three. By donating these electrons, they acquire a stable shell of the nearest inert gas:
$Ca^0 hspace{10pt}-2bar{e}rightarrow Ca^{+2}$
$overbrace{1s^22s^22p^63s^23p^64s^2}hspace{10pt}-2bar{e}rightarrowoverbrace{1s^22s^22p^63s^2 3p^6}$
Thus, metals in chemical reactions are reducing agents - they acquire a positive oxidation state. This is their fundamental difference from non-metal elements.
Definition
The ability of an atom of an element to shift electrons from a chemical bond to itself is called electronegativity .
Due to low electronegativity values, metals give up electrons more easily than attract them, and therefore exhibit reducing properties.
The words “metal” and “non-metal” apply not only to chemical elements, but also to simple substances. For example, when we say that a simple substance is a metal, we mean not only that it consists of atoms of a metal element, but also a certain commonality of physical (metallic luster, plasticity) and chemical (reducing agent) properties. The metallic properties of simple substances decrease when moving through a period from left to right, and through a group - from bottom to top. Metallic properties are most pronounced in the elements of the main subgroup of Group I of the Periodic Table - the alkali metals. Their atoms give up a valence electron so easily that in nature these elements are found exclusively in the form of compounds.
Crystal lattice and metal bond
Metals have a metallic crystal lattice, in the nodes of which individual atoms are located. They weakly hold valence electrons, which for this reason move freely throughout the entire volume of the metal, forming a single electron cloud and are equally attracted by all atoms. Such a bond is called metallic .
The general properties of metals - plasticity, ability to reflect light, thermal and electrical conductivity - are explained by the peculiarities of their structure. With strong pressure, a piece of metal changes shape - some of the atoms are displaced, but do not crumble: the common electron cloud firmly holds all the atoms together. In an electric field, free electrons begin to move in a certain direction; such ordered movement of electrons is called electric current.
The more free electrons there are in a metal and the stronger the vibrations of atoms located at lattice sites, the faster the temperature equalization occurs throughout the entire piece of metal, that is, the greater its thermal conductivity. Therefore, the relative values of thermal and electrical conductivity for many metals are close.
Physical properties of metals
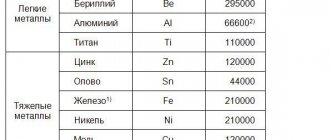
Physical state and melting points . The melting temperatures of metals vary over a very wide range. The most fusible of metals, mercury, is a liquid at room temperature. Gallium metal melts from the heat of the human body. Of the metals widely used in technology, the most fusible are tin and lead. Tungsten, from which light bulb filaments are made, has the highest melting point. Metals with a melting point above $1000^oC$ are usually called refractory.
mercury gallium tungsten
Coloring . Among metals, few have a characteristic color. “Gold, due to its fairly yellow color and brilliant lightness, is different from other metals,” wrote Mikhail Vasilyevich Lomonosov. Copper is pink-red, silver and platinum are white, and the alkali metal cesium is pale yellow. It is difficult to find words to describe the color of other metals. They all seem gray to us with one or another barely noticeable tint.