AISI 304
Designation according to international standards
| International standard | American ASTM A240 | European EN 10088-2 | Russian GOST 5632-72 |
| Brand designation | AISI 304 | 1.4301 | 08Х18Н10 |
| 12Х18Н9 |
Applicable standards and approvals
AMS 5513 ASTM A 240 ASTM A 666
Classification
corrosion-resistant, heat-resistant steel
Application
- Household items
- Sinks
- Frames for metal structures in the construction industry
- Kitchen utensils and catering equipment
- Dairy equipment, brewing
- Welded structures
- Tanks on ships and land tankers for food, beverages and some chemicals
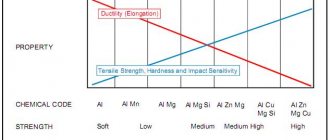
Typically, steel manufacturers divide the grade into three main classes (grades) according to their drawing ability:
- AISI 304 – Basic grade
- AISI 304 DDQ (Normal and deep drawing) – Deep drawing grade
- AISI 304 DDS (Extra deep drawing) – Extra deep drawing grade
Main characteristics
- good overall corrosion resistance
- good ductility
- excellent weldability
Chemical composition (% by weight)
| standard | brand | C | Si | Mn | P | S | Cr | Ni |
| ASTM A240 | AISI 304 | ≤0.080 | ≤0.75 | ≤2.0 | ≤0.045 | ≤0.030 | 18.00 – 20.00 | 8.00 – 10.50 |
Mechanical properties
| AISI 304 | Tensile strength (σв), N/mm² | Yield strength(σ0.2), N/mm² | Yield strength(σ1.0), N/mm² | Relative elongation (σ), % | Brinell Hardness (HB) | Rockwell Hardness (HRB) |
| According to EN 10088-2 | ≥520 | ≥210 | ≥250 | ≥45 | – | – |
| According to ASTM A 240 | ≥515 | ≥205 | – | ≥40 | 202 | 85 |
Mechanical properties at high temperatures
All these values refer only to AISI 304
.
Physical properties
| Physical properties | Legend | Unit | Temperature | Meaning |
| Density | d | – | 4°C | 7.93 |
| Melting temperature | °C | 1450 | ||
| Specific heat | c | J/kg.K | 20°C | 500 |
| Thermal expansion | k | W/mK | 20°C | 15 |
| Average coefficient of thermal expansion | α | 10 -6 .K -1 | 0-100°C 0-200°C | 17.5 18 |
| Electrical resistivity | ρ | Ωmm 2 /m | 20°C | 0.80 |
| Magnetic permeability | μ | at 0.80 kA/m DC or h/h AC | 20°C μ μ discharge air | 1.02 |
| Elastic modulus | E | MPa x 10 3 | 20°C | 200 |
Corrosion resistance
304 steels have good resistance to general corrosive environments, but are not recommended where there is a risk of intergranular corrosion. They are well suited for use in fresh water and urban and rural environments. In all cases, regular cleaning of external surfaces is necessary to maintain their original condition.
304 steels have good resistance to various acids:
- phosphoric acid in all concentrations at ambient temperature,
- nitric acid up to 65% at temperatures 20°C – 50°C,
- formic and lactic acid at room temperature,
- acetic acid at a temperature of 20°C – 50°C.
They are recommended for the production of equipment in contact with cold or hot food products: wine, beer, milk (fermented milk products), alcohol, natural fruit juices, syrups, molasses, etc.
Acidic environments
| Temperature, °C | 20 | 80 | ||||||||||
| Concentration, % by weight | 10 | 20 | 40 | 60 | 80 | 100 | 10 | 20 | 40 | 60 | 80 | 100 |
| Sulfuric acid | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Nitric acid | 2 | 1 | 2 | |||||||||
| Phosphoric acid | 2 | 1 | 2 | |||||||||
| Formic acid | 1 | 2 | 2 | 1 | ||||||||
Code: 0 = high degree of protection – Corrosion rate less than 100 µm/year 1 = partial protection – Corrosion rate from 100 to 1000 µm/year 2 = no protection – Corrosion rate more than 1000 µm/year
Atmospheric influences
Comparison of 304
grades with other metals in various environments (Corrosion rate calculated for 10-year exposure).
| Environment | Corrosion rate (μm/year) | ||
| AISI 304 | Aluminum-3S | Carbon steel | |
| Rural | 0.0025 | 0.025 | 5.8 |
| Marine | 0.0076 | 0.432 | 34.0 |
| Industrial Marine | 0.0076 | 0.686 | 46.2 |
Resistant to corrosion in boiling chemicals
| Boiling medium | Metal condition | Corrosion rate (mm/year) |
| 20% acetic acid | Regular metal Welded | * (With a sample thickness of 0.8 mm and a press diameter of 20 mm) |
| AISI 430 | 2.05 mm | |
| AISI 304 | 2.0 mm |
*Limiting drawing ratio – limiting drawing ratio
Physical meaning of conductivity
The use of metal conductors has a long history. Scientists and engineers working in fields of science and technology that use electricity have long decided on materials for wires, terminals, contacts, etc. A physical quantity called electrical conductivity helps determine the most electrically conductive metal in the world.
The concept of conductivity is the inverse of electrical resistance. The quantification of conductivity is related to the unit of resistance, which is measured in Ohms in the International System of Units (SI). The SI unit is siemens. The Russian designation for this unit is Cm, the international designation is S. An electrical conductivity of 1 Cm has a section of an electrical network with a resistance of 1 Ohm.
Resistivity of metals and alloys (at 20° C)
| Substance | Resistivity µOhm • mm 2 /m |
| Aluminum | 0,028 |
| Tungsten | 0,055 |
| Iron | 0,098 |
| Gold | 0,023 |
| Constantan | 0,44−0,52 |
| Brass | 0,025−0,06 |
| Manganin | 0,42−0,48 |
| Copper | 0,0175 |
| Molybdenum | 0,057 |
| Nikelin | 0,39−0,45 |
| Nickel | 0,100 |
| Tin | 0,115 |
| Mercury | 0,958 |
| Lead | 0,221 |
| Silver | 0,016 |
| Tantalum | 0,155 |
| Fechral | 1,1−1,3 |
| Chromium | 0,027 |
| Zinc | 0,059 |
| Substance | TO | Substance | TO |
| Aluminum | 0,0042 | Tin | 0,0042 |
| Tungsten | 0,0048 | Platinum | 0,004 |
| Constantan | 0,00002 | Mercury | 0,0009 |
| Brass | 0,001 | Lead | 0,004 |
| Copper | 0,0043 | Silver | 0,0036 |
| Manganin | 0,00003 | Steel | 0,006 |
| Molybdenum | 0,0033 | Tantalum | 0,0031 |
| Nickel | 0,005 | Chromium | 0,006 |
| Nikelin | 0,0001 | Fechral | 0,0002 |
| Nichrome | 0,0001 | Zinc | 0,004 |
Alloys (composition in%):
- Constantan (58.8 Cu, 40 Ni, 1.2 Mn)
- Manganin (85 Cu, 12 Mn, 3 Ni)
- Nickel silver (65 Cu, 20 Zn, 15 Ni)
- Nickelin (54 Cu, 20 Zn, 26 Ni)
- Nichrome (67.5 Ni, 15 Cr, 16 Fe, 1.5 Mn)
- Rheonate (84Cu, 12Mn, 4 Zn)
- Fechral (80 Fe, 14 Cr, 6 Al)
Nichrome resistivity
Every body through which an electric current is passed automatically exhibits a certain resistance to it. The property of a conductor to resist electric current is called electrical resistance.
Let's consider the electronic theory of this phenomenon. When moving along a conductor, free electrons constantly encounter other electrons and atoms on their way. By interacting with them, a free electron loses part of its charge. Thus, the electrons encounter resistance from the conductor material. Each body has its own atomic structure, which provides different resistance to electric current. The unit of resistance is considered to be Ohm. The resistance of materials is designated R or r.
The lower the resistance of the conductor, the easier it is for electric current to pass through this body. And vice versa: the higher the resistance, the worse the body conducts electric current.
The resistance of each individual conductor depends on the properties of the material from which it is made. To accurately characterize the electrical resistance of a particular material, the concept of resistivity (nichrome, aluminum) was introduced. Specific resistance is considered to be the resistance of a conductor up to 1 m long, the cross-section of which is 1 square meter. mm. This indicator is denoted by the letter p. Each material used in the production of a conductor has its own resistivity. For example, consider the resistivity of nichrome and fechral (more than 3 mm):
- Х15Н60 — 1.13 Ohm*mm/m
- Х23У5Т — 1.39 Ohm*mm/m
- Х20Н80 — 1.12 Ohm*mm/m
- ХН70У — 1.30 Ohm*mm/m
- ХН20УС — 1.02 Ohm*mm/m
The resistivity of nichrome and fechral indicates their main area of application: the manufacture of thermal devices, household appliances and electric heating elements for industrial furnaces.
Since nichrome and fechral are mainly used in the production of heating elements, the most common products are nichrome thread, tape, strip X15N60 and X20N80, as well as fechral wire X23Yu5T.
How does the electrical conductivity of different metals differ?
The electronic theory of electrical conductivity of metals was developed in the research of Paul Drude. He was able to discover such a property as resistance, which is observed when electric current passes through a conductor. In the future, this will make it possible to classify different substances according to their conductivity level. From the results obtained, it is easy to understand which metal is suitable for the manufacture of a particular cable. This is a very important point, since incorrectly selected material can cause a fire as a result of overheating from the passage of excess voltage current.
Silver metal has the highest electrical conductivity. At a temperature of +20 degrees Celsius, it is 63.3 * 104 centimeters-1. But making wiring from silver is very expensive, since it is a rather rare metal, which is used mainly for the production of jewelry and decorative items or bullion coins.
The metal with the highest electrical conductivity among all elements of the base group is copper. Its indicator is 57*104 centimeters-1 at a temperature of +20 degrees Celsius. Copper is one of the most common conductors used for household and industrial purposes. It withstands constant electrical loads well, is durable and reliable. The high melting point allows you to work for a long time in a heated state without problems.
In terms of abundance, only aluminum can compete with copper, which ranks fourth in electrical conductivity after gold. It is used in networks with low voltage, as it has almost half the melting point of copper and is not able to withstand extreme loads. The further distribution of places can be found by looking at the table of electrical conductivity of metals.
It is worth noting that any alloy has much lower conductivity than the pure substance. This is due to the merging of the structural network and, as a consequence, disruption of the normal functioning of electrons. For example, in the production of copper wire, a material with an impurity content of no more than 0.1% is used, and for some types of cable this indicator is even stricter - no more than 0.05%. All given indicators are the electrical conductivity of metals, which is calculated as the ratio between the current density and the magnitude of the electric field in the conductor.
Steel Specifications
Before considering the resistivity of steel in detail, you should familiarize yourself with its basic physical and mechanical properties. Due to its qualities, this material is widely used in the manufacturing sector and other areas of people’s lives and activities.
Steel is an alloy of iron and carbon, contained in an amount not exceeding 1.7%. In addition to carbon, steel contains a certain amount of impurities - silicon, manganese, sulfur and phosphorus. In terms of its qualities, it is much better than cast iron; it can easily be hardened, forged, rolled and other types of processing. All types of steels are characterized by high strength and ductility.
According to its purpose, steel is divided into structural, instrumental, and also with special physical properties. Each of them contains a different amount of carbon, thanks to which the material acquires certain specific qualities, for example, heat resistance, heat resistance, resistance to rust and corrosion.
A special place is occupied by electrical steels, produced in sheet format and used in the production of electrical products. To obtain this material, silicon is doped, which can improve its magnetic and electrical properties.
In order for electrical steel to acquire the necessary characteristics, certain requirements and conditions must be met. The material must be easily magnetized and remagnetized, that is, have high magnetic permeability. Such steels have good magnetic induction, and their magnetization reversal occurs with minimal losses.
The dimensions and weight of magnetic cores and windings, as well as the efficiency of transformers and their operating temperature depend on compliance with these requirements. The fulfillment of the conditions is influenced by many factors, including the resistivity of steel.
Classical theory of electrical conductivity of metals
The basic principles of the theory of electrical conductivity of metals contain six points. First: a high level of electrical conductivity is associated with the presence of a large number of free electrons. Second: electric current arises through external influence on the metal, during which electrons move from random motion to ordered motion.
Third: the strength of the current passing through a metal conductor is calculated according to Ohm's law. Fourth: different numbers of elementary particles in the crystal lattice lead to unequal resistance of metals. Fifth: electric current in the circuit arises instantly after the start of exposure to electrons. Sixth: as the internal temperature of the metal increases, the level of its resistance also increases.
The nature of the electrical conductivity of metals is explained by the second point of the provisions. In a quiet state, all free electrons rotate chaotically around the nucleus. At this moment, the metal is not able to independently reproduce electrical charges. But as soon as you connect an external source of influence, the electrons instantly line up in a structured sequence and become carriers of electric current. With increasing temperature, the electrical conductivity of metals decreases.
This is due to the fact that the molecular bonds in the crystal lattice weaken, elementary particles begin to rotate in an even more chaotic order, so the formation of electrons in a chain becomes more complicated. Therefore, it is necessary to take measures to prevent overheating of the conductors, as this negatively affects their performance properties. The mechanism of electrical conductivity of metals cannot be changed due to the current laws of physics. But it is possible to neutralize negative external and internal influences that interfere with the normal course of the process.
Resistivity and other indicators
The value of electrical resistivity is the ratio of the electric field strength in the metal and the current density flowing in it. For practical calculations, the formula is used: in which ρ is the resistivity of the metal (Ohm*m), E is the electric field strength (V/m), and J is the electric current density in the metal (A/m 2 ). At very high electric field strength and low current density, the resistivity of the metal will be high.
There is another quantity called electrical conductivity, the inverse of resistivity, indicating the degree to which a material conducts electric current. It is determined by the formula and expressed in units of S/m - siemens per meter.
Resistivity is closely related to electrical resistance. However, they have differences among themselves. In the first case, this is a property of the material, including steel, and in the second case, the property of the entire object is determined. The quality of a resistor is influenced by a combination of several factors, primarily the shape and resistivity of the material from which it is made. For example, if a thin and long wire was used to make a wirewound resistor, then its resistance will be greater than that of a resistor made from a thick and short wire of the same metal.
Another example is resistors made of wires of the same diameter and length. However, if in one of them the material has a high resistivity, and in the other it is low, then, accordingly, the electrical resistance in the first resistor will be higher than in the second.
Knowing the basic properties of the material, you can use the resistivity of steel to determine the resistance value of a steel conductor. For calculations, in addition to the electrical resistivity, you will need the diameter and length of the wire itself. Calculations are performed using the following formula: , in which R is the resistance of the conductor (Ohm), ρ is the resistivity of steel (Ohm*m), L corresponds to the length of the wire, A is its cross-sectional area.
There is a dependence of the resistivity of steel and other metals on temperature. In most calculations, room temperature is used - 20 0 C. All changes under the influence of this factor are taken into account using the temperature coefficient.
High conductivity materials
The most widespread materials of high conductivity include copper and aluminum (Superconducting materials, which have a typical resistance 10-20 times lower than ordinary conductive materials (metals), are discussed in the section Superconductivity).
Copper
The advantages of copper, which ensure its widespread use as a conductor material, are as follows:
- low resistivity;
- sufficiently high mechanical strength;
- corrosion resistance is satisfactory in most applications;
- good workability: copper is rolled into sheets, strips and drawn into wire, the thickness of which can be increased to thousandths of a millimeter;
- relative ease of soldering and welding.
Copper is most often obtained by processing sulfide ores. After a series of ore smelting and roasting with intense blasting, copper intended for electrical purposes must undergo a process of electrolytic purification.
Copper grades M1 and M0 are most often used as conductor material. M1 grade copper contains 99.9% Cu, and in the total amount of impurities (0.1%) oxygen should be no more than 0.08%. The presence of oxygen in copper worsens its mechanical properties. The best mechanical properties are found in M0 grade copper, which contains no more than 0.05% impurities, including no more than 0.02% oxygen.
Copper is a relatively expensive and scarce material, so it is increasingly being replaced by other metals, especially aluminum.
In some cases, alloys of copper with tin, silicon, phosphorus, beryllium, chromium, magnesium, and cadmium are used. Such alloys, called bronzes, with the correct composition, have significantly higher mechanical properties than pure copper.
Aluminum
Aluminum is the second most important conductor material after copper. This is the most important representative of the so-called light metals: the density of cast aluminum is about 2.6, and rolled aluminum is 2.7 Mg/m3. Thus, aluminum is approximately 3.5 times lighter than copper. The temperature coefficient of expansion, specific heat capacity and heat of fusion of aluminum are greater than those of copper. Due to the high values of specific heat capacity and heat of fusion, heating aluminum to the melting point and transferring it to a molten state requires more heat than heating and melting the same amount of copper, although the melting point of aluminum is lower than that of copper.
Aluminum has lower properties compared to copper - both mechanical and electrical. With the same cross-section and length, the electrical resistance of an aluminum wire is 1.63 times greater than that of a copper wire. It is very important that aluminum is less scarce than copper.
For electrical purposes, aluminum containing no more than 0.5% impurities, grade A1, is used. Even purer AB00 grade aluminum (no more than 0.03% impurities) is used for the manufacture of aluminum foil, electrodes and housings of electrolytic capacitors. Aluminum of the highest purity AB0000 has an impurity content of no more than 0.004%. Additives of Ni, Si, Zn or Fe at a content of 0.5% reduce the γ of annealed aluminum by no more than 2-3%. A more noticeable effect is exerted by Cu, Ag and Mg impurities, which, at the same mass content, reduce γ aluminum by 5-10%. Ti and Mn greatly reduce the electrical conductivity of aluminum.
Aluminum oxidizes very actively and becomes covered with a thin oxide film with high electrical resistance. This film protects the metal from further corrosion.
Aluminum alloys have increased mechanical strength. An example of such an alloy is Aldrey , containing 0.3-0.5% Mg, 0.4-0.7% Si and 0.2-0.3% Fe. In aldrey, the Mg2Si compound is formed, which imparts high mechanical properties to the alloy.
Iron and steel
Iron (steel), as the cheapest and most accessible metal, which also has high mechanical strength, is of great interest for use as a conductor material. However, even pure iron has a significantly higher resistivity compared to copper and aluminum; ρ steel, i.e. iron mixed with carbon and other elements is even higher. Ordinary steel has low corrosion resistance: even at normal temperatures, especially in conditions of high humidity, it quickly rusts; As the temperature rises, the corrosion rate increases sharply. Therefore, the surface of steel wires must be protected by a layer of more resistant material. Zinc coating is usually used for this purpose.
In some cases, to reduce the consumption of non-ferrous metals, the so-called bimetal . It is steel coated on the outside with a layer of copper, with both metals connected to each other firmly and continuously.
Sodium
Sodium metal is a very promising conductor material. Sodium can be obtained by electrolysis of molten sodium chloride NaCl in virtually unlimited quantities. From a comparison of the properties of sodium with the properties of other conductor metals, it is clear that the resistivity of sodium is approximately 2.8 times greater than ρ of copper and 1.7 times greater than ρ of aluminum, but due to the extremely low density of sodium (its density is almost 9 times less than the density of copper), a wire made of sodium for a given conductivity per unit length should be significantly lighter than a wire made of any other metal. However, sodium is extremely active chemically (it oxidizes intensely in air and reacts violently with water), which is why the sodium wire must be protected with a sealing sheath. The sheath must give the wire the necessary mechanical strength, since sodium is very soft and has a low tensile strength during deformation.
Literature on conductor resistivity
- Kuznetsov M.I., “Fundamentals of Electrical Engineering” - 9th edition, revised - Moscow: Higher School, 1964 - 560 p.
- Bachelis D. S., Belorussov N. I., Saakyan A. E. Electrical cables, wires and cords. Directory. - M.: Energy, 1971.
- Gershun A.L. Cable // Encyclopedic Dictionary of Brockhaus and Efron: in 86 volumes (82 volumes and 4 additional). - St. Petersburg, 1890-1907.
- R. Lakernik, D. Charlet. From copper to glass // Science and life. - 1986. - Issue. 08. - pp. 50-54, 2-3 pages, color insert.
TOEE CHPP RiEKT Metrology Real physics Superconductivity Theory of conductivity
Did you know,
How is Olbers' paradox resolved? (The photometric paradox, Olbers' paradox, is one of the paradoxes of cosmology, which consists in the fact that in a Universe uniformly filled with stars, the brightness of the sky (including the night sky) should be approximately equal to the brightness of the solar disk. This should take place because, according to any direction of the sky, the ray of vision will sooner or later hit the surface of the star. In other words, Olbers' paradox is that if the Universe is infinite, then we will not see a black sky, since the radiation of distant stars will be summed up with the radiation of nearby ones, and the sky should have an average temperature of the photospheres stars. When light is absorbed by interstellar matter, it will heat up to the temperature of stellar photospheres and radiate as brightly as stars. However, the phenomenon of “light fatigue” comes into play, discovered by Edwin Hubble, who showed that the farther a galaxy is located from us, the more the light of its radiation turns red, that is, the photons seem to “get tired”, giving up their energy to the interstellar medium.At very large distances, galaxies are visible only in the radio range, since their light has completely lost energy while traveling through the vast expanses of the Universe. Read more in the FAQ on ethereal physics.
Metals with high electrical conductivity
The electrical conductivity of alkali metals is at a high level, since their electrons are weakly attached to the nucleus and easily line up in the desired sequence. But this group is characterized by low melting points and enormous chemical activity, which in most cases does not allow their use for the manufacture of wires.
Metals with high electrical conductivity when opened are very dangerous for humans. Touching a bare wire will result in an electrical burn and a powerful discharge to all internal organs. This often results in instant death. Therefore, special insulating materials are used for the safety of people.
Depending on the application, they can be solid, liquid or gaseous. But all types are designed to do one thing—isolating electrical current within a circuit so that it cannot affect the outside world. The electrical conductivity of metals is used in almost all areas of modern human life, so ensuring safety is a top priority.
It surprises no one today that when we touch the switch button, we see a light bulb come on. Often we don’t even think that all such actions are based on a whole series. One of these extremely interesting phenomena is the electrical conductivity of metals, which ensures the flow of electric current.
To begin with, we should probably decide what we are talking about. So, electrical conductivity is the ability of a substance to transmit. Moreover, different substances have this ability to varying degrees. Based on the degree of electrical conductivity, substances are divided into conductors, semiconductors and dielectrics.
If you look at the experimental data obtained by researchers during the study of electric current, it becomes clear that the conductivity of metals is the highest. This is also confirmed by everyday practice, when metal wires are used to transmit electric current. Metals are primarily conductors of electric current. And the explanation for this can be found in the electronic theory of metals.
According to the latter, the conductor is a crystal lattice, the nodes of which are occupied by atoms. They are located very densely and are connected with neighboring similar atoms, so they remain practically at the nodes of the crystal lattice. The same cannot be said about electrons located on the outer shells of atoms. These electrons are free to move randomly, forming what is called an “electron gas.” The electronic conductivity of metals is based on such electrons.
As evidence that the nature of electric current is due to electrons, we can recall the experiment of the German physicist Rikke, carried out in 1901. He took two copper and one aluminum cylinders with carefully polished ends, placed one on top of the other and passed an electric current through them. According to the experimenter, if the electrical conductivity of metals is due to atoms, then a transfer of matter would occur. However, after passing an electric current for a year, the mass of the cylinders did not change.
From this result it was concluded that the electrical conductivity of metals is caused by some particles inherent in all conductors. The electron, which had already been discovered by that moment, was precisely suited to this role. Subsequently, several more ingenious experiments were carried out, and they all confirmed that electric current is caused by the movement of electrons.
In accordance with modern ideas about metals, ions are located at its nodes, and electrons move relatively freely between them. It is the large number of such electrons that ensures the high electrical conductivity of metals. When there is a small amount at the ends of the conductor, these free electrons begin to move, which causes the flow of electric current.
It should be noted here that conductivity strongly depends on temperature. Thus, with increasing temperature, the conductivity of metals decreases, and vice versa, it increases with decreasing temperature, up to At the same time, it should be remembered that although all metals have conductivity, its value is different for each of them. Of the most widely used metals used in electrical engineering, copper has the best conductivity.
So, the above material gives an idea of what the electrical conductivity of metals is, explains the nature of electric current and explains what causes it. A description of the crystal lattice of metals and the influence of some external factors on conductivity is given.
Electronic conductivity of metals
At the beginning of the 20th century, the classical electronic theory of conductivity of metals was created (P. Drude, 1900, H. Lorenz, 1904), which provided a simple and visual explanation of most of the electrical and thermal properties of metals. Let's consider some provisions of this theory.
Free electrons
The metal conductor consists of:
1) positively charged ions oscillating around the equilibrium position, and
2) free electrons capable of moving throughout the entire volume of the conductor.
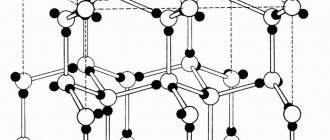
Thus, the electrical properties of metals are determined by the presence of free electrons in them with a concentration of the order of 1028 m–3, which approximately corresponds to the concentration of atoms. These electrons are called conduction electrons. They are formed by removing their valence electrons from metal atoms. Such electrons do not belong to any particular atom and are able to move throughout the entire volume of the body. In a metal, in the absence of an electric field, conduction electrons move chaotically and collide, most often with ions of the crystal lattice (Fig. 1). The collection of these electrons can be approximately considered as a kind of electron gas, subject to the laws of an ideal gas. The average speed of thermal motion of electrons at room temperature is approximately 105 m/s.
Picture 1
Electric current in metals
The ions of the metal crystal lattice do not take part in the creation of current. Their movement during the passage of current would mean the transfer of matter along the conductor, which is not observed. For example, in the experiments of E. Riecke (1901), the mass and chemical composition of the conductor did not change when current passed for a year.
Experimental proof that the current in metals is created by free electrons was given in experiments by L.I. Mandelstam and N.D. Papaleksi (1912, the results were not published), as well as T. Stewart and R. Tolman (1916). They discovered that when a rapidly rotating coil suddenly stops, an electric current arises in the coil conductor, created by negatively charged particles - electrons.
Consequently, electric current in metals is the directed movement of free electrons.
Since electric current in metals is formed by free electrons, the conductivity of metallic conductors is called electronic conductivity.
Electric current in metals arises under the influence of an external electric field. The conduction electrons located in this field are acted upon by an electric force, imparting to them an acceleration directed in the direction opposite to the field strength vector. As a result, the electrons acquire a certain additional speed (it is called drift). This speed increases until the electron collides with an atom in the metal crystal lattice. During such collisions, electrons lose their excess kinetic energy, transferring it to ions. Then the electrons are again accelerated by the electric field, again decelerated by ions, etc. The average electron drift speed is very small, about 10–4 m/s.
Current propagation speed and drift speed are not the same thing. The speed of current propagation is equal to the speed of propagation of the electric field in space, i.e. 3⋅108 m/s.
When colliding with ions, conduction electrons transfer part of the kinetic energy to the ions, which leads to an increase in the energy of motion of the ions of the crystal lattice, and, consequently, to heating of the conductor.
Metal resistance
The resistance of metals is explained by collisions of conduction electrons with ions of the crystal lattice. In this case, obviously, the more often such collisions occur, i.e., the shorter the average free travel time of an electron between collisions τ, the greater the resistivity of the metal.
In turn, time τ depends on the distance between the lattice ions, the amplitude of their vibrations, the nature of the interaction of electrons with ions and the speed of thermal motion of electrons. As the temperature of the metal increases, the amplitude of ion vibrations and the speed of thermal motion of electrons increase. The number of crystal lattice defects also increases. All this leads to the fact that as the temperature of the metal increases, collisions of electrons with ions will occur more often, i.e. time τ decreases, and the resistivity of the metal increases.