Tungsten
- the most refractory of metals. Only the non-metallic element, carbon, has a higher melting point. Under standard conditions it is chemically resistant. The name Wolframium was transferred to the element from the mineral wolframite, known back in the 16th century. called lat. Spuma lupi (“wolf foam”) or German. Wolf Rahm (“wolf cream”, “wolf cream”). The name was due to the fact that tungsten, accompanying tin ores, interfered with the smelting of tin, turning it into foam of slag (“devours tin like a wolf devours a sheep”).
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Silver
— structure and physical properties
STRUCTURE
Tungsten crystal has a body-centered cubic lattice. Tungsten crystals in the cold are characterized by low plasticity, therefore, during the process of pressing the powder, they practically do not change their basic shape and size, and the compaction of the powder occurs mainly through the relative movement of particles.
In a body-centered cubic tungsten cell, the atoms are located at the vertices and in the center of the cell, i.e. There are two atoms per cell. The bcc structure is not the closest packing of atoms. The compactness coefficient is 0.68. Tungsten space group Im3m.
PROPERTIES
Tungsten is a shiny light gray metal that has the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but this cannot be firmly stated yet - the lifetime of seaborgium is very short). Melting point - 3695 K (3422 °C), boils at 5828 K (5555 °C). The density of pure tungsten is 19.25 g/cm³. It has paramagnetic properties (magnetic susceptibility 0.32·10−9). Brinell hardness 488 kg/mm², electrical resistivity at 20 °C - 55·10−9 Ohm·m, at 2700°C - 904·10−9 Ohm·m. The speed of sound in annealed tungsten is 4290 m/s. Is paramagnetic.
Tungsten is one of the heaviest, hardest and most refractory metals. In its pure form, it is a silvery-white metal, similar to platinum, at a temperature of about 1600 °C it is easily forged and can be drawn into a thin thread.
RESERVES AND PRODUCTION
The tungsten Clarke of the earth's crust is (according to Vinogradov) 1.3 g/t (0.00013% of the content in the earth's crust). Its average content in rocks, g/t: ultrabasic - 0.1, basic - 0.7, intermediate - 1.2, acidic - 1.9.
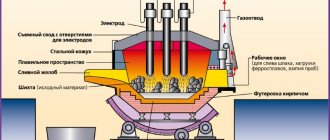
The process of obtaining tungsten goes through the substage of separation of trioxide WO3 from ore concentrates and subsequent reduction to metal powder with hydrogen at a temperature of about 700 °C. Due to the high melting point of tungsten, powder metallurgy methods are used to obtain a compact form: the resulting powder is pressed, sintered in a hydrogen atmosphere at a temperature of 1200-1300 ° C, then an electric current is passed through it. The metal is heated to 3000 °C, and sintering occurs into a monolithic material. For subsequent purification and obtaining a single-crystalline form, zone melting is used.
Tungsten ore mining
The tungsten content in the earth's crust is just over one ten-thousandth of a percent, which makes it a fairly rare natural resource. It is not found in its pure form, so minerals such as wolframite and scheelite are used to extract it. These are tungsten ores that contain, in addition to the base metal, a number of impurities.
In the mines
The underground method of mining ores containing tungsten involves the sequential collapse of horizontal layers of the mine with further accumulation of material in spent blocks (the so-called “magazine”). Then the collected workings are loaded onto transport and removed to the surface.
In the quarries
In them, the extraction of tungsten ores is carried out by open-pit mining. By collapsing external soil, loading it onto transport systems and sending it for processing.
ORIGIN
Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Wolframite (iron and manganese tungstate nFeWO4 * mMnWO4 - ferberite and hübnerite, respectively) and scheelite (calcium tungstate CaWO4) are of industrial importance. Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
Kazakhstan, China, Canada and the USA have the largest reserves; deposits are also known in Bolivia, Portugal, Russia, Uzbekistan and South Korea. World tungsten production is 49-50 thousand tons per year, including 41 in China, 3.5 in Russia; Kazakhstan 0.7, Austria 0.5. Main exporters of tungsten: China, South Korea, Austria. Main importers: USA, Japan, Germany, UK. There are also tungsten deposits in Armenia and other countries.
Being in nature
Place of Birth
The geological structure of the earth's crust is such that the largest deposits of tungsten ores are located in the Alps, Himalayas, and mountain ranges of the Pacific Ocean region. These are the territories of Kazakhstan (the largest deposit is Upper Kairakty), China (the most productive deposit is Jianshi), Canada (Tangsten deposit) and the USA (significant reserves have been explored in the Climax deposit).
There are also areas of concentration of wolframites and scheelites in Bolivia, Portugal, Great Britain, Turkey, Russia, Uzbekistan, South Korea, and Australia.
In space
Progress does not stand still, and the earth's resources are distributed extremely unevenly and are quite limited. The exploration of outer space, which has made it possible to take samples from the surfaces of a number of celestial bodies of nearby objects in the Solar System, gives every reason to assume the presence of a huge amount of minerals on asteroids, comets and planets.
This opens up very attractive prospects for their future development. It is assumed that asteroids contain a huge amount of minerals, and in very high concentrations. Including tungsten. Due to the fact that some of these celestial bodies rotate close to the Earth, the prospects for their exploration become very, very tempting.
Governments of a number of countries, international space communities and private agencies are actively creating a legal framework, developing programs, and sending missions. Thus, Luxembourg was the first to pass a law allowing private mining in space. Not only the leading space powers of the world are active in this issue, but also Japan, India, Australia, and Israel. Active research is being carried out on the surface of the Moon, Mars, and Venus.
It is difficult to give any assessment to these efforts, since many organizational, technical and financial problems stand in the way. Although many experts consider it possible to mine tungsten in space in the 21st century.
APPLICATION
The refractoriness and ductility of tungsten make it indispensable for incandescent filaments in lighting fixtures, as well as in picture tubes and other vacuum tubes. Due to its high density, tungsten is the basis of heavy alloys that are used for counterweights, armor-piercing cores of sub-caliber and swept-fin projectiles of artillery guns, cores of armor-piercing bullets and high-speed gyroscope rotors to stabilize the flight of ballistic missiles (up to 180 thousand rpm).
Tungsten is used as electrodes for argon-arc welding. Alloys containing tungsten are characterized by heat resistance, acid resistance, hardness and abrasion resistance. They are used to make surgical instruments (amaloy alloy), tank armor, shells of torpedoes and shells, the most important parts of aircraft and engines, and containers for storing radioactive substances. Tungsten is an important component of the best grades of tool steels. Tungsten is used in high-temperature vacuum resistance furnaces as heating elements. An alloy of tungsten and rhenium is used in such furnaces as a thermocouple.
For mechanical processing of metals and non-metallic structural materials in mechanical engineering (turning, milling, planing, chiselling), well drilling, and in the mining industry, hard alloys and composite materials based on tungsten carbide are widely used (for example, pobedit, consisting of WC crystals in a cobalt matrix; grades widely used in Russia - VK2, VK4, VK6, VK8, VK15, VK25, T5K10, T15K6, T30K4), as well as mixtures of tungsten carbide, titanium carbide, tantalum carbide (TT grades for particularly difficult processing conditions, for example, chiselling and planing forgings made of heat-resistant steels and rotary hammer drilling of strong materials). Widely used as an alloying element (often together with molybdenum) in steels and iron-based alloys. High-alloy steel, classified as “high-speed”, with a marking starting with the letter P, almost always contains tungsten. (P18, P6M5. from rapid - fast, speed).
Tungsten sulfide WS2 is used as a high-temperature (up to 500 °C) lubricant. Some tungsten compounds are used as catalysts and pigments. Tungstate single crystals (lead, cadmium, calcium tungstates) are used as scintillation detectors of X-rays and other ionizing radiation in nuclear physics and nuclear medicine.
Tungsten ditelluride WTe2 is used to convert thermal energy into electrical energy (thermo-emf about 57 μV/K). Artificial radionuclide 185W is used as a radioactive tracer in substance research. Stable 184W is used as a component of uranium-235 alloys used in solid-phase nuclear rocket engines because it is the only common tungsten isotope that has a low thermal neutron capture cross section (about 2 barn).
Tungsten - W
| Molecular weight | 183.84 g/mol |
| origin of name | lat. Spuma lupi (“wolf foam”) or German. Wolf Rahm (“wolf cream”, “wolf cream”) |
| IMA status | confirmed in 2011 |
Applications of tungsten alloys
Tungsten is capable of forming alloys with cobalt, iron, nickel and other metals. As already mentioned, tungsten can react with various chemical elements and thereby eliminate the negative effects of some of them (sulfur, phosphorus) in alloys. As a result, we obtain tungsten alloys - hard, chemically resistant and elastic.
For example, alloys of tungsten with boron and carbon are very close in hardness to diamonds, the hardest minerals.
Some alloys are suitable for producing parts that can be used to operate at elevated temperatures. Tungsten-molybdenum alloys are used to produce jet nozzles and wire. The military industry actively uses heavy tungsten alloys to create tanks, grenades, weapons, and shells. Tungsten has become an excellent replacement for lead in this area.
We have listed the main areas and applications of tungsten, and, as you can see, this chemical element has remained in demand for many years in a wide variety of industries. The unique properties of tungsten indicate that in the future it may become doubly popular and will be used in completely new areas.
PHYSICAL PROPERTIES
| Mineral color | grey |
| Stroke color | white |
| Transparency | opaque |
| Shine | metal |
| Cleavage | No |
| Hardness (Mohs scale) | 7,5 |
| Strength | malleable |
| Kink | jagged |
| Density (measured) | 19.3 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Magnetism | paramagnetic |