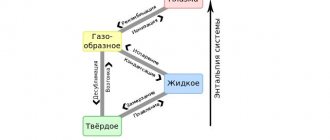
Each metal and their alloys have different properties. One of these properties is the melting point. Each metal melts at a different temperature. All that is needed to transfer a substance from a solid to a liquid state is a heat source that will heat the metal to a certain temperature.
Since each metal has a different melting point, it is possible to determine which metal is less resistant to temperature or more. So the most fusible metal is mercury , it is ready to go into a liquid state at a temperature of 39 degrees Celsius . But tungsten (which is what tungsten electrodes for argon welding are actually made of) will melt only when the temperature reaches 3422 degrees Celsius .
For alloys such as steel and others, it is quite difficult to determine the temperature at which they will melt. The whole complexity is in their composition... Since the composition is different, the melting point is different. Typically, alloys are given a temperature range at which they will melt. In general, the melting point of metals is an interesting topic.
What is melting point
Each metal has unique properties, and this list includes its melting point. When melting, the metal changes from one state to another, namely, from solid to liquid. To fuse a metal, you need to bring heat closer to it and heat it to the required temperature - this process is called the melting point. At the moment when the temperature reaches the desired level, it can still remain in a solid state. If you continue the impact, the metal or alloy will begin to melt.
Melting and boiling are not the same thing. The point at which a substance transitions from solid to liquid is often referred to as the melting point of the metal. In the molten state, the molecules do not have a specific arrangement, but attraction holds them close together; in liquid form, the crystalline body leaves volume, but the shape is lost.
During boiling, the volume is lost, the molecules interact very weakly with each other, move chaotically in different directions, and separate from the surface. Boiling point is the process by which the pressure of metal vapor is equal to the pressure of the external environment.
In order to simplify the difference between critical heating points, we have prepared a simple table for you:
| Property | Melting temperature | Boiling temperature |
| Physical state | The alloy transforms into a melt, the crystalline structure is destroyed, and granularity passes through | Transforms into a gas state, some molecules can fly away outside the melt |
| Phase transition | Equilibrium between solid and liquid | Pressure equilibrium between metal vapor and air |
| Influence of external pressure | No changes | There are changes, the temperature decreases with discharge |
Melting point prediction (Lindemann criterion)
An attempt to predict the melting point of crystalline materials was made in 1910 by Frederick Lindemann [6]. The idea was the observation that the average amplitude of thermal fluctuations increases with increasing temperature. Melting begins when the vibration amplitude becomes large enough for neighboring atoms to partially occupy the same space.
The Lindeman criterion states that melting is expected when the rms value of the oscillation amplitude exceeds a threshold value.
The melting point of crystals is described quite well by the Lindemann formula [7]:
Tλ=xm29ℏ2MkBθrs2{displaystyle T_{lambda }={frac {x_{m}^{2}}{9hbar ^{2}}}Mk_{B}theta r_{s}^{2}}
where rs{displaystyle r_{s}} is the average radius of the unit cell, θ{displaystyle theta } is the Debye temperature, and the parameter xm{displaystyle x_{m}} for most materials varies in the range of 0.15-0.3.
Melting point - calculation
Lindemann's formula served as a theoretical basis for melting for almost a hundred years, but was not developed due to low accuracy.
Test Aggregate states of matter. Melting and solidification of crystalline bodies, grade 8
Test Aggregate states of matter. Melting and solidification of crystalline solids grade 8 with answers. The test includes 18 tasks.
1. The state of aggregation of a substance is its presence in the form
1) a solid body 2) a liquid body 3) a gaseous body 4) any of these three bodies
2. In what state of aggregation can iron and mercury exist?
1) iron in a solid, mercury in a liquid 2) both iron and mercury in a liquid 3) both iron and mercury in a solid 2) both substances can be in any state of aggregation
3. What determines what specific state of aggregation a substance is in?
1) on the number and composition of molecules 2) on the arrangement, interaction and movement of molecules 3) on the arrangement and composition of molecules 4) on the interaction and number of molecules
4. What happens to the molecules of a substance during its transition from one state of aggregation to another?
1) the composition of the molecules changes 2) the shape and composition of the molecules change 3) the molecules remain the same 4) the molecules acquire different sizes
5. Melting is
1) melting of snow or ice 2) liquefaction of a substance when it receives heat 3) transition when a substance receives energy from a solid to a liquid state
6. The temperature at which a substance melts is called
1) temperature of transition to the liquid state 2) melting temperature 3) melting temperature
7. Melting point of zinc is 420 °C. What state is this metal in if its temperature is 410 °C (No. 1)? 430 °C (No. 2)?
1) No. 1 – solid, No. 2 – liquid 2) No. 1 – liquid, No. 2 – solid 3) No. 1 and No. 2 – liquid 4) No. 1 and No. 2 – solid
8. Hardening is
1) release of energy by a substance and transformation into another substance 2) transition of a substance from a liquid to a solid state 3) freezing of water
9. How does the internal energy of a substance change during melting? When curing?
1) decreases during melting, increases during solidification 2) does not change 3) increases in both cases 4) increases during melting, decreases during solidification
10. The melting point of steel is 1500 °C. At what temperature does it harden?
1) at temperatures below 1500 °C 2) at 1500 °C 3) at temperatures above 1500 °C 4) at any temperature, if it releases energy
11. From what metal - aluminum, copper or steel - should a melting vessel be made in order to melt lead in it?
1) from aluminum 2) from copper 3) from steel 4) from any of the above
12. Zinc and iron plates fell into a vessel with molten aluminum. Which one will melt?
1) zinc 2) iron 3) none 4) both
13. What state will mercury and sodium be in at room temperature (20 °C)?
1) in solid 2) in liquid 3) mercury - in liquid, sodium in solid 4) mercury in solid, sodium in liquid
14. The figure shows a graph of heating and melting of snow and heating of the water obtained from it. Which part of the graph corresponds to snow melting? Approximately how long did it last? To what temperature did the water heat up in 5 minutes?
1) aircraft; 3.5 min; 30 °C 2) BC; 2 minutes; 30 °C 3) AB; 1.5 min; 30 °C 4) Sun; 3.5 min; 40 °C
15. Water from a room with a temperature of 25 °C was taken out into 30-degree frost, where it turned into ice. A graph of changes in its temperature and ice is shown in the figure. Which part of it corresponds to the solidification of water? What does the DE region indicate?
1) aircraft; about the ice reaching the ambient temperature and stopping its change 2) AB; about equalizing the temperatures of ice and air 3) CD; that the ice temperature became equal to 30 ° C
16. What happens to the temperature of a substance during its melting?
1) it decreases 2) it increases 3) remains constant
17. Which of the given graphs of changes in the temperature of a substance corresponds to the process of its solidification, which to heating without transition to another state of aggregation?
1) №3; №1 2) №2; №3 3) №1; №2 4) №2; №1
18. Which part of the graphs No. 1 and No. 2 of the temperature change of the substance shown here corresponds to its solidification?
1) AB 2) FK 3) EF 4) CD
Answers to the test Aggregate states of matter. Melting and solidification of crystalline bodies 8th grade 1-4 2-4 3-2 4-3 5-3 6-2 7-1 8-2 9-4 10-2 11-4 12-1 13-3 14-2 15 -1 16-3 17-4 18-2
PDF printable version Physics test Aggregate states of matter. Melting and solidification of crystalline bodies, grade 8 (170 Kb)
Graphical representation of melting and solidification processes
A graph of the melting and solidification of crystalline solids provides a visual representation of the time dependence of these phase transitions.
Rice. 2. Water-ice melting and solidification graph.
Ordinary water is a good example to illustrate the phenomena discussed. In the presented graph, time t is plotted along the abscissa axis, and temperature is plotted along the ordinate axis. Let initially, at the moment of time t = 0, when the temperature of the ice (crystal) was equal to -400C, the heat supply - heating - will begin. Let us next consider the time dependence of the temperature dependence T(t):
- In section AB, from -400C to 00C (melting temperature of ice), ice exists in crystalline form;
- Section BC - the melting stage occurs, both ice and water are present. The temperature remains constant, equal to 00C;
- CD - at point C melting has ended, there is only a liquid phase - water;
- DE - at point D heating has stopped, cooling occurs up to point E, i.e. up to a temperature of 00C. Only liquid water is present;
- EF - at point E, solidification begins, ice crystals appear, but at the same time there is also a liquid phase. The temperature remains constant, equal to 00C;
- FK - at point F complete solidification has occurred, only ice remains in crystalline form, the temperature of which gradually decreases.
At what temperature does it melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
Different substances have different melting points. Theoretically, metals are divided into:
- Low-melting - temperatures up to 600 degrees Celsius are sufficient to obtain a liquid substance.
- Medium melting - requires a temperature of 600 to 1600 ⁰C.
- Refractory are metals that require temperatures above 1600 ⁰C to melt.
Melting iron
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray – the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White – the temperature reaches 1350 degrees. It is poured into molds at 1450.
Important! The melting rates of a metal such as cast iron are 400 degrees lower compared to steel. This significantly reduces energy costs during processing.
Melting steel
Melting steel at a temperature of 1400 °C
Steel is an alloy of iron mixed with carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Reference! Steel melts at 1400 degrees Celsius.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Metal melting process
This process refers to the transition of a substance from a solid to a liquid state.
When the melting point is reached, the metal can be in either a solid or liquid state; further increase will lead to the complete transition of the material into a liquid. The same thing happens during solidification - when the melting point is reached, the substance will begin to transition from a liquid to a solid state, and the temperature will not change until complete crystallization.
It should be remembered that this rule applies only to pure metal. Alloys do not have a clear temperature boundary and undergo state transitions in a certain range:
- Solidus is the temperature line at which the most fusible component of the alloy begins to melt.
- Liquidus is the final melting point of all components, below which the first alloy crystals begin to appear.
It is impossible to accurately measure the melting point of such substances; the point of transition of states is indicated by a numerical interval.
Depending on the temperature at which metals begin to melt, they are usually divided into:
- Low-melting, up to 600 °C. These include tin, zinc, lead and others.
- Medium melting, up to 1600 °C. Most common alloys, and metals such as gold, silver, copper, iron, aluminum.
- Refractory, over 1600 °C. Titanium, molybdenum, tungsten, chromium.
There is also a boiling point - the point at which the molten metal begins to transition into a gaseous state. This is a very high temperature, typically 2 times the melting point.
Effect of pressure
The melting temperature and the equal solidification temperature depend on pressure, increasing with its increase.
This is due to the fact that with increasing pressure the atoms come closer to each other, and in order to destroy the crystal lattice they need to be moved away. At increased pressure, greater thermal energy is required and the corresponding melting temperature increases. There are exceptions when the temperature required to transform into a liquid state decreases with increased pressure. Such substances include ice, bismuth, germanium and antimony.
Metal crystal lattices
In its ideal form, it is generally accepted that metals have a cubic lattice (real substances may have flaws). There are equal distances between molecules horizontally and vertically.
A solid substance is characterized by constancy:
- shapes, the object retains linear dimensions in different conditions;
- volume, the object does not change the amount of substance it occupies;
- mass, the amount of a substance expressed in grams (kilograms, tons);
- density, unit volume contains constant mass.
When transitioning into a liquid state, having reached a certain temperature, the crystal lattices are destroyed. Now we can’t talk about constancy of form. The liquid will take the form in which it is poured.
When evaporation occurs, only the mass of the substance remains constant. Gas will take up the entire volume that will be provided to it. Here we cannot say that density is a constant value.
When liquids combine, the following options are possible:
- Liquids completely dissolve in one another, as do water and alcohol. The concentration of substances will be the same throughout the entire volume.
- Liquids are stratified by density, the connection occurs only at the interface. It is only temporarily possible to obtain a mechanical mixture. Mix liquids with different properties. An example is oil and water.
Metals form alloys in the liquid state. To obtain an alloy, each of the components must be in a liquid state. With alloys, phenomena of complete dissolution of one in another are possible. Options cannot be excluded when the alloy will be obtained only as a result of intensive mixing. In this case, the quality of the alloy is not guaranteed, so they try not to mix components that do not allow obtaining stable alloys.
The resulting substances, soluble in each other, when solidified, form crystal lattices of a new type. Define:
- Heliocentered crystal lattices are also called body-centered. In the middle there is a molecule of one substance, and four more molecules of another are located around it. It is customary to call such lattices loose, since the bonds between metal molecules in them are weaker.
- Face-centered crystal lattices form compounds in which the component molecules are located on the faces. Metallurgists call such crystalline alloys dense. In reality, the density of the alloy can be higher than that of each of the components included in the composition (alchemists of the Middle Ages were looking for options for alloys in which the density would correspond to the density of gold).
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Determination of specific heat of fusion
The specific heat of fusion (designated by the Greek letter “lambda” - λ) is a physical quantity equal to the amount of heat (in joules) that must be transferred to a solid body weighing 1 kg in order to completely transform it into the liquid phase. The formula for the specific heat of fusion looks like this:
$$ λ ={Q over m}$$
Where:
m is the mass of the melting substance;
Q is the amount of heat transferred to the substance during melting.
Values for different substances are determined experimentally.
Knowing λ, we can calculate the amount of heat that must be imparted to a body of mass m for its complete melting:
$$Q={λ*m}$$
What does melting temperature depend on?
For different substances, the temperature at which the structure is completely reconstructed to the liquid state is different. If we take into account metals and alloys, then it is worth noting the following points:
- Metals are not often found in their pure form. The temperature directly depends on its composition. As an example, we will indicate tin, to which other substances (for example, silver) can be added. Impurities make the material more or less resistant to heat.
- There are alloys that, due to their chemical composition, can transform into a liquid state at temperatures above one hundred and fifty degrees. There are also alloys that can “hold” when heated to three thousand degrees and above. Taking into account the fact that when the crystal lattice changes, the physical and mechanical qualities change, and operating conditions can be determined by the heating temperature. It is worth noting that the melting point of a metal is an important property of a substance. An example of this is aviation equipment.
Heat treatment, in most cases, almost does not change the resistance to heat. The only sure way to increase resistance to heat is to make changes to the chemical composition; this is why steel is alloyed.
Crystallization
When the temperature of a liquid decreases, its molecules become less mobile. And the attractive forces that hold molecules in a certain strict order, characteristic of a solid, increase.
If a liquid substance is cooled to a certain temperature, it will solidify. The process of phase transition from liquid to solid is called crystallization
. Unlike melting, when a substance receives heat, during crystallization it releases it, and its temperature decreases.
The temperature at which this process occurs is called the crystallization temperature
. For a pure substance, the melting point is equal to the crystallization temperature.
Like melting, crystallization also occurs gradually. Likewise, a liquid and a solid will have the same temperature until the entire substance solidifies.
Liquid tin melted by a soldering iron solidifies and becomes solid when we remove the soldering iron. Molten liquid metal poured into molds solidifies as the temperature drops.
We observe crystallization in nature every year, when water in reservoirs freezes at low temperatures and snowflakes fall to the ground instead of raindrops.
Why does a solid become liquid?
Heating a solid body leads to an increase in the kinetic energy of atoms and molecules, which at normal temperatures are located clearly at the nodes of the crystal lattice, which allows the body to maintain a constant shape and size. When certain critical speed values are reached, atoms and molecules begin to leave their places, bonds are broken, the body begins to lose its shape - it becomes liquid. The melting process does not occur abruptly, but gradually, so that for some time the solid and liquid components (phases) are in equilibrium. Melting refers to endothermic processes, that is, those that occur with the absorption of heat. The opposite process, when a liquid solidifies, is called crystallization.
Rice. 1. Transition of the solid, crystalline state of a substance into the liquid phase.
It was discovered that until the end of the melting process the temperature does not change, although heat is constantly supplied. There is no contradiction here, since the incoming energy during this period of time is spent on breaking the crystalline bonds of the lattice. After the destruction of all bonds, the influx of heat will increase the kinetic energy of the molecules, and consequently, the temperature will begin to rise.
Rice. 2. Graph of body temperature versus heating time.
Melting
melting of propane, melting of lead Melting
is the process of transition of a body from a crystalline solid state to a liquid, that is, the transition of a substance from one state of aggregation to another.
Melting occurs with the absorption of specific heat of fusion
and is a first-order phase transition, which is accompanied by an abrupt change in heat capacity at a specific temperature point of transformation for each substance - the melting point.
The ability to melt refers to the physical properties of a substance
At normal pressure, the highest melting point
among metals it has tungsten (3422 °C), among simple substances - carbon (according to various sources, 3500 - 4500 °C) and among arbitrary substances - tantalum-hafnium carbide Ta4HfC5 (3942 °C). We can assume that helium has the lowest melting point: at normal pressure it remains liquid at arbitrarily low temperatures.
Many substances at normal pressure do not have a liquid phase. When heated, they immediately transform into a gaseous state by sublimation.
- 1 Melting of mixtures and solid solutions
- 2 Melting kinetics 2.1 Nature of melting
- 2.2 Melting dynamics
- 2.3 Melting in two-dimensional systems
Melting mixtures and solid solutions
Alloys generally do not have a specific melting point; the process of their melting occurs in a finite temperature range. In the temperature-relative concentration diagrams, there is a finite region of coexistence of the liquid and solid states, limited by the liquidus and solidus curves. A similar situation occurs in the case of many solid solutions.
Amorphous bodies also do not have a fixed melting temperature; they turn into a liquid state gradually, softening as the temperature rises.
Melting kinetics
Technically, the melting of a substance is carried out by supplying thermal energy from outside the sample (external heating, for example, in a thermal furnace) or directly throughout its entire volume (internal heating, for example, resistive heating when passing current through the sample, or induction heating in a high-frequency electromagnetic field ). The melting method does not affect the main characteristics of the process - temperature and latent heat of fusion, but determines the external picture of melting, for example, the appearance of a quasi-liquid layer on the surface of the sample during external heating.
It is believed that melting is characterized by the loss of long-range orientational interatomic order in the crystal with a transition to “liquid-like” or “gas-tight” disorder.
Nature of melting
Let us first explain why, at a certain temperature, a body prefers to break some of the interatomic bonds and go from an ordered state (crystal) to a disordered state (liquid).
As is known from thermodynamics, at a fixed temperature a body tends to minimize free energy. At low temperatures, the second term (the product of temperature and entropy) is insignificant, and as a result, everything comes down to minimizing ordinary energy.
In this case, ordinary energy will increase slightly, but entropy will also increase greatly, which will result in a decrease in free energy.
Melting dynamics
In dynamics, melting occurs as follows. As the temperature of a body increases, the amplitude of thermal vibrations of its molecules increases, and from time to time structural defects of the lattice appear in the form of atomic jumps, the growth of dislocations and other disturbances of the crystal lattice.
Each such defect, the emergence and movement of dislocations, requires a certain amount of energy, since it is accompanied by the rupture of some interatomic bonds. The stage of birth and accumulation of defects is called the premelting stage. In addition, at this stage, as a rule, with external heating, a quasi-liquid layer appears on the surface of the body.
It is believed that at a certain temperature the concentration of defects becomes so large that it leads to a loss of orientational order in the sample.
Temperature fluctuations of atoms in the crystal lattice Behavior of the liquid after the crystal passes through the melting point, as, on average, constant breaks and restorations of intercluster and intracluster interatomic bonds for a given temperature (short thickened segments - broken bonds)
Due to the fact that the mechanism of thermal destruction of a crystal due to the formation of defects and the growth of dislocations, which occurs over a wide temperature range, does not lead to a phase transformation of the 1st kind, that is, to a jump in the thermodynamic characteristics of a substance at a specific temperature point for each substance, Lindeman developed simple ideas about the course of the melting process, according to which the amplitude of vibration of particles at the melting point increases so much that it becomes comparable to the interatomic distance in the crystal lattice and leads to destruction of the lattice and loss of orientational interatomic order. In fact, this “melting factor” is the basis of most models with the determining role of the repulsive part of the pair interaction potential and the imposition of conditions for the transition from order to “liquid-like” or “gas-tight” disorder, calculated by Monte Carlo and molecular dynamics methods. However, it was found that at the melting point the root mean square displacement of atoms from equilibrium is only about 1/8 of the interatomic distance, which excludes the Lindemann model, that is, the collision of atoms, as a “melting factor”. In this case, the energy of the atoms is significantly lower than the potential energy of atomization of the crystal lattice.
Further studies showed that the dynamics of melting of a crystalline body, as a phase transformation of the 1st kind, is determined (in contrast to the model of accumulation of defects and dislocations) by a “catastrophic” (crash) conformational transformation of the structure of a group of atoms, accompanied by the destruction of interatomic bonds when overcoming a potential barrier with the expenditure of a constant amount of energy, lower than the lattice atomization energy, and equal to the specific heat of fusion.
This mechanism leads to an experimentally confirmed cluster structure of a bound (condensed) liquid state with a constant (for a given temperature) average number of breaking and reforming intercluster and intracluster interatomic bonds, which determines the mobility (fluidity) of the liquid.
With increasing temperature, the number of atoms in clusters decreases and at the boiling point the substance passes into a monoatomic (monomolecular) gaseous state.
Melting in two-dimensional systems
In two-dimensional or quasi-two-dimensional systems, the crystal is a much more shaky object than in the three-dimensional case, namely, a two-dimensional crystal has no long-range positional order. For comparison, in the one-dimensional case, a crystal at a finite temperature cannot be stable at all.
As it turned out, this leads to the melting of a two-dimensional crystal occurring in two stages. First, the crystal goes into the so-called hexatic phase, in which the short-range positional order is lost, but the orientational order is preserved, and then the orientational order is also lost and the body becomes liquid.
Notes
- ↑
S. T. Zhukov Chemistry 8-9 grades, Chapter 1. Basic ideas and concepts of chemistry - ↑
The scatter of experimental data is apparently associated with the graphite-carbyne phase transition and different heating rates during measurements. Klimovsky I. I., Markovets V. V.The influence of the graphite-carbyne phase transition on the emissivity of graphite samples when heated to temperatures of 3000 K or more // International Scientific Journal for Alternative Energy and Ecology. - 2007. - No. 6 (50). — P. 50-59.
- ↑
Meyer K. Physico-chemical crystallography, M., "Metallography", 1972
- ↑
LindemannF.A. // Phys.Z., 1910, v.11, p.609 - ↑
Wood WW, Jacobson JD Preliminary Results from a Recalculation of the Monte Carlo Equation of State of Hard Spheres // J. Chem. Phys.. - 1957. - No. 27. - P. 1207. - DOI:10.1063/1.1743956. - ↑
Alder BJ, Wainwright TE Phase Transition in Elastic Disks // Phys. Rev.. - 1962. - No. 127. - P. 359. - DOI:10.1103/PhysRev.127.359.
- ↑
Hoover WG, Gray SG, Johnson KW Thermodynamic Properties of the Fluid and Solid Phases for Inverse Power Potentials // J. Chem. Phys.. - 1971. - No. 55. - P. 1128. - DOI:10.1063/1.1676196.
- ↑
Pines D. Elementary excitations in solids. M., Mir, 1965.
Links
- Surface premelting of ice
- Melting of two-dimensional crystals
melting water, melting ice, melting metals, melting propane, melting lead
Melting Information About
Melting
Melting Melting You are viewing the subject Melting what, Melting who, Melting description
There are excerpts from wikipedia on this article and video
Our site has a system in search engine function. Above: “What were you looking for?” you can query everything in the system with the box. Welcome to our simple, stylish and fast search engine, which we have prepared to provide you with the most accurate and up-to-date information.
The search engine designed for you brings you the most current and accurate information with a simple design and fast functioning system. You can find almost any information you are looking for on our website.
At the moment we serve only in English, Turkish, Russian, Ukrainian, Kazakh and Belarusian. New languages will be added to the system very soon.
Lives of Famous People gives you information, images and videos about hundreds of topics such as politicians, government figures, doctors, internet sites, plants, technological vehicles, cars, etc.
Melting point table
To find out what temperature is needed to melt metals, a table of increasing temperature indicators will help.
| Element or connection | Required temperature conditions |
| Lithium | +18°С |
| Potassium | +63.6°С |
| Indium | +156.6°С |
| Tin | +232°С |
| Thallium | +304°С |
| Cadmium | +321°С |
| Lead | +327°С |
| Zinc | +420°С |
Melting table for medium-melting metals and alloys.
| Element or alloy | Temperature |
| Magnesium | +650°С |
| Aluminum | +660°С |
| Barium | +727°С |
| Silver | +960°С |
| Gold | +1063°С |
| Manganese | +1246°С |
| Copper | +1083°С |
| Nickel | +1455°С |
| Cobalt | +1495°С |
| Iron | +1539°С |
| Durali | +650°С |
| Brass | +950…1050°С |
| Cast iron | +1100…1300°С |
| Carbon steels | +1300…1500°С |
| Nichrome | +1400°С |
Melting table for refractory metals and alloys.
| Item name | Temperature |
| Titanium | +1680°С |
| Platinum | +1769.3°С |
| Chromium | +1907°С |
| Zirconium | +1855°С |
| Vanadium | +1910°С |
| Iridium | +2447°С |
| Molybdenum | +2623°С |
| Tantalum | +3017°С |
| Tungsten | +3420°С |
Classification of metals by melting point
In physics, the transition of a solid into a liquid state is characteristic only of substances with a crystalline structure. The melting point of metals is often indicated by a range of values; for alloys, it is difficult to accurately determine heating to the boundary phase state. For pure elements, every degree matters, especially if these are fusible elements,
doesn't matter. The summary table of t indicators is usually divided into 3 groups. In addition to low-melting elements, which heat up to a maximum of +600°C, there are refractory elements that can withstand heating above +1600°C, and medium-melting ones. This group includes alloys that form a melt pool at temperatures from +600 to 1600°C.
Low-melting metals
Low-melting metals have a melting point below 600°C. These are zinc, tin, bismuth. Such metals can be melted at home by heating them on the stove, or using a soldering iron. Low-melting metals are used in electronics and technology to connect metal elements and wires for the movement of electric current. The melting point of tin is 232 degrees, and zinc is 419.
Table of low-melting metals and alloys (up to 600C o)
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tin | Sn | 232 Co | 2600 So |
| Lead | Pb | 327 Co | 1750 So |
| Zinc | Zn | 420 Co | 907 Co |
| Potassium | K | 63.6 Co | 759 Co |
| Sodium | Na | 97.8 Co | 883 Co |
| Mercury | Hg | — 38.9 Co | 356.73 Co |
| Cesium | Cs | 28.4 Co | 667.5 Co |
| Bismuth | Bi | 271.4 Co | 1564 Co |
| Palladium | Pd | 327.5 Co | 1749 Co |
| Polonium | Po | 254 Co | 962 Co |
| Cadmium | Cd | 321.07 Co | 767 Co |
| Rubidium | Rb | 39.3 Co | 688 Co |
| Gallium | Ga | 29.76 So | 2204 Co |
| Indium | In | 156.6 Co | 2072 Co |
| Thallium | Tl | 304 Co | 1473 Co |
| Lithium | Li | 18.05 So | 1342 Co |
Medium melting metals
Medium-melting metals begin to transform from solid to liquid at temperatures from 600°C to 1600°C. They are used to make slabs, reinforcements, blocks and other metal structures suitable for construction. This group of metals includes iron, copper, aluminum, and they are also part of many alloys. Copper is added to alloys of precious metals such as gold, silver, and platinum. 750 gold consists of 25% alloy metals, including copper, which gives it a reddish tint. The melting point of this material is 1084 °C. And aluminum begins to melt at a relatively low temperature of 660 degrees Celsius. This is a lightweight, ductile and inexpensive metal that does not oxidize or rust, therefore it is widely used in the manufacture of tableware. The melting point of iron is 1539 degrees. This is one of the most popular and affordable metals, its use is widespread in the construction and automotive industries. But due to the fact that iron is subject to corrosion, it must be additionally processed and covered with a protective layer of paint, drying oil, or prevent moisture from entering.
Table of medium-melting metals and alloys (from 600C to 1600C)
| Item name | Latin designation | Temperature | |
| Melting | Boiling | ||
| Aluminum | Al | 660 Co | 2519 So |
| Germanium | Ge | 937 Co | 2830 Co |
| Magnesium | Mg | 650 Co | 1100 So |
| Silver | Ag | 960 Co | 2180 Co |
| Gold | Au | 1063 Co | 2660 Co |
| Copper | Cu | 1083 Co | 2580 Co |
| Iron | Fe | 1539 So | 2900 So |
| Silicon | Si | 1415 Co | 2350 Co |
| Nickel | Ni | 1455 Co | 2913 Co |
| Barium | Ba | 727 Co | 1897 Co |
| Beryllium | Be | 1287 Co | 2471 Co |
| Neptunium | Np | 644 Co | 3901.85 Co |
| Protactinium | Pa | 1572 Co | 4027 Co |
| Plutonium | Pu | 640 Co | 3228 Co |
| Actinium | Ac | 1051 Co | 3198 Co |
| Calcium | Ca | 842 Co | 1484 Co |
| Radium | Ra | 700 Co | 1736.85 So |
| Cobalt | Co | 1495 Co | 2927 Co |
| Antimony | Sb | 630.63 Co | 1587 Co |
| Strontium | Sr | 777 Co | 1382 Co |
| Uranus | U | 1135 Co | 4131 Co |
| Manganese | Mn | 1246 Co | 2061 Co |
| Konstantin | 1260 So | ||
| Duralumin | Alloy of aluminum, magnesium, copper and manganese | 650 Co | |
| Invar | Nickel iron alloy | 1425 Co | |
| Brass | Copper and zinc alloy | 1000 Co | |
| Nickel silver | Alloy of copper, zinc and nickel | 1100 So | |
| Nichrome | Alloy of nickel, chromium, silicon, iron, manganese and aluminum | 1400 So | |
| Steel | Iron-carbon alloy | 1300 Co – 1500 Co | |
| Fechral | Alloy of chromium, iron, aluminum, manganese and silicon | 1460 So | |
| Cast iron | Iron-carbon alloy | 1100 Co – 1300 Co | |
Refractory metals
The temperature of refractory metals is above 1600°C. These are tungsten, titanium, platinum, chromium and others. They are used as light sources, machine parts, lubricants, and in the nuclear industry. They are used to make wires, high-voltage wires, and are used to melt other metals with a lower melting point. Platinum begins to transition from solid to liquid at a temperature of 1769 degrees, and tungsten at a temperature of 3420°C.
Mercury is the only metal that is in a liquid state under normal conditions, namely, normal atmospheric pressure and average ambient temperature. The melting point of mercury is minus 39°C. This metal and its vapors are poisonous, so it is used only in closed containers or in laboratories. A common use of mercury is as a thermometer to measure body temperature.
Each metal and alloy has its own unique set of physical and chemical properties, not least of which is the melting point. The process itself means the transition of a body from one state of aggregation to another, in this case, from a solid crystalline state to a liquid one. To melt a metal, it is necessary to apply heat to it until the melting temperature is reached. With it, it can still remain in a solid state, but with further exposure and increased heat, the metal begins to melt. If the temperature is lowered, that is, some of the heat is removed, the element will harden.
The highest melting point among metals belongs to tungsten: it is 3422C o, the lowest is mercury: the element melts at -39C o. As a rule, it is not possible to determine the exact value for alloys: it can vary significantly depending on the percentage of components. They are usually written as a number interval.
Table of refractory metals and alloys (over 1600C o)
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tungsten | W | 3420 Co | 5555 Co |
| Titanium | Ti | 1680 So | 3300 So |
| Iridium | Ir | 2447 Co | 4428 Co |
| Osmium | Os | 3054 Co | 5012 Co |
| Platinum | Pt | 1769.3 Co | 3825 Co |
| Rhenium | Re | 3186 Co | 5596 Co |
| Chromium | Cr | 1907 Co | 2671 Co |
| Rhodium | Rh | 1964 Co | 3695 Co |
| Ruthenium | Ru | 2334 Co | 4150 Co |
| Hafnium | Hf | 2233 Co | 4603 Co |
| Tantalum | Ta | 3017 Co | 5458 Co |
| Technetium | Tc | 2157 Co | 4265 Co |
| Thorium | Th | 1750 So | 4788 Co |
| Vanadium | V | 1910 Co | 3407 Co |
| Zirconium | Zr | 1855 Co | 4409 Co |
| Niobium | Nb | 2477 Co | 4744 Co |
| Molybdenum | Mo | 2623 Co | 4639 Co |
| Hafnium carbides | 3890 Co | ||
| Niobium carbides | 3760 Co | ||
| Titanium carbides | 3150 Co | ||
| Zirconium carbides | 3530 Co | ||
Specific heat of fusion of certain substances
Information on specific heat values for a specific substance can be found in book reference books or in electronic versions on Internet resources. They are usually presented in table form:
Specific heat of fusion of substances
| Substance | 105 * J/kg | kcal/kg | Substance | 105 * J/kg | kcal/kg |
| Aluminum | 3,8 | 92 | Mercury | 0,1 | 3,0 |
| Iron | 2,7 | 65 | Lead | 0,3 | 6,0 |
| Ice | 3,3 | 80 | Silver | 0,87 | 21 |
| Copper | 1,8 | 42 | Steel | 0,8 | 20 |
| Naphthalene | 1,5 | 36 | Zinc | 1,2 | 28 |
| Tin | 0,58 | 14 | Platinum | 1,01 | 24,1 |
| Paraffin | 1,5 | 35 | Gold | 0,66 | 15,8 |
One of the most refractory substances is tantalum carbide - TаC. It melts at a temperature of 39900C. TAC coatings are used to protect metal molds in which aluminum parts are cast.
Rice. 3. Metal melting process.
Which metal has the highest melting point
Tungsten is the most refractory metal, 3422 °C (6170 °F).
A hard, refractory, fairly heavy material of light gray color that has a metallic sheen. It is difficult to machine. At room temperature it is quite fragile and breaks. The fragility of the metal is associated with contamination with carbon and oxygen impurities.
Note! Technically, pure metal at temperatures above four hundred degrees Celsius becomes very ductile. Demonstrates chemical inertness and is reluctant to react with other elements. It occurs in nature in the form of such complex minerals as: hübnerite, scheelite, ferberite and wolframite.
Tungsten can be obtained from its ore, through complex chemical processing, as a powder. Using pressing and sintering, it is used to create parts of regular shape and bars.
Tungsten is an extremely resistant element to any temperature influences. For this reason, tungsten could not be softened for more than a hundred years. There was no furnace that could heat up to several thousand degrees Celsius. Scientists have been able to prove that this is the most refractory metal. Although there is an opinion that seaborgium, according to some theoretical data, has greater refractoriness, this is only an assumption, since it is a radioactive element and has a short lifespan.