Complete annealing of aluminum and aluminum alloys
After complete annealing, all aluminum alloys - both thermally hardenable and non-thermally hardenable - obtain a state that is the softest, most ductile and most favorable for plastic deformation.
The international designation for this condition is the letter “O”. Sometimes this letter
"O" is confused with
the number
"0".
In domestic standards for aluminum products there is a state of simply “annealing” and this state is designated by the letter “M”. In terms of the meaning and mechanical properties of alloys in this state, this “simple” annealing is complete
annealing, as understood in international standards.
Annealing temperature
A reduction or complete removal of strain hardening from cold plastic deformation (cold-frettening or cold-working) is achieved by heating to a temperature from 260 to 440 °C. This is true for both thermally hardenable and non-thermally hardenable aluminum alloys.
The rate of softening of cold-worked material strongly depends on temperature. Therefore, the time required to fully anneal a given aluminum alloy with a given degree of work hardening can vary from several hours at low temperatures to several seconds at high temperatures.
Application of metal in industrial production
Under natural conditions, aluminum tends to form a thin oxide film, which prevents reactions with water and nitric acid (without heating). When the film is destroyed as a result of contact with alkalis, the chemical element acts as a reducing agent.
In order to prevent the formation of an oxide film, other metals (gallium, tin, indium) are added to the alloy. The metal is practically not subject to corrosion processes. It is a popular material in various industries.
Aluminum and its alloys are in great demand in various spheres of human life.
- Aluminum is considered a popular material for making tableware and the main raw material for the aviation and space industries. The excellent electrical conductivity of the metal allows it to be used for sputtering conductors in microelectronics.
- The property of aluminum and its alloys to become brittle at low temperatures allows it to be used in cryogenic technology. Reflectivity and low cost, ease of vacuum deposition make aluminum an indispensable material for the manufacture of mirrors.
- The application of metal to the surface of parts of turbines and oil platforms makes steel alloys resistant to corrosion. Metal sulfide is used to produce hydrogen sulfide, and pure aluminum is used as a reducing agent for rare alloys from oxides.
- The chemical element is used as a component of compounds, for example, in aluminum bronzes and magnesium alloys. Along with other materials, it is used for the manufacture of spirals in electric heating devices. Metal compounds are widely used in glass making.
- Currently, pure aluminum is rarely used as a material for jewelry, but its alloy with gold, which has a special shine and sparkle, is gaining popularity. In Japan, metal is used instead of silver to make jewelry.
- Aluminum is registered as an additive in the food industry. Aluminum beer cans have been a popular beverage packaging since the 1960s. The technological line provides for the production of containers of 0.33 and 0.5 liters. The packaging has the same diameter and differs only in height.
- The main advantage of packaging over glass is the possibility of recycling the material.
- Cans for beer (carbonated drinks) can withstand pressure up to 6 atmospheres, have a dome-shaped, thick bottom and thin walls. Features of the manufacturing technology by drawing ensure the structural strength and reliable performance properties of the container.
What is the purpose of annealing - this is the annealing temperature
If the purpose of annealing is simply to remove strain hardening, then heating to a temperature of about 345 °C will be quite sufficient. If it is necessary to remove strengthening from heat treatment or even simply from cooling from the hot treatment temperature, then special heat treatment is needed to obtain a structure with the release of the strengthening phase in the form of large and separate particles. This heat treatment is complete annealing: holding at temperatures from 415 to 440 °C and slow cooling at a rate of about 30 °C per hour to 260 °C.
High rates of diffusion of alloying elements in aluminum, which are characteristic of such a high temperature, the duration of exposure and slow cooling ensure maximum coalescence (coarsening) of the particles of the strengthening phase, which results in the material - aluminum alloy - minimal hardness.
Melting aluminum
Influence of alloying elements and impurities
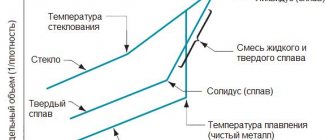
Adding other elements to aluminum, including alloying ones, reduces its melting point, or more precisely, the beginning of its melting. Thus, for some cast aluminum alloys with a high content of silicon and magnesium, the melting point decreases to almost 500 °C. In general, the concept of “melting point” applies only to pure metals and other crystalline substances. Alloys do not have a specific melting point: the process of their melting (and solidification) occurs in a certain temperature range.
Figure 4 - Change in the specific volume of pure metal (aluminum) and the alloy of this metal (aluminum alloy) [4]
Melting temperature ranges
The table below presents the liquidus and solidus temperatures of some commercial wrought alloys. It must be borne in mind that the concepts of solidus and liquidus temperatures are defined for equilibrium transformations of the liquid phase into the solid phase and vice versa, that is, for infinite duration of the processes. In practice, corrections must be made taking into account the rate of heating or cooling.
Melting silumin
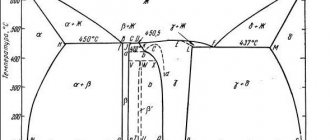
Not all alloys have a range between solidus and liquidus temperatures. Such alloys are called eutectic. For example, in an aluminum alloy containing 12.5% silicon, the liquidus and solidus points are reduced to a point: this alloy, like pure metals, has not an interval, but a melting point. This point and temperature is called eutectic. This alloy belongs to the famous cast aluminum-silicon alloys - silumins with a narrow solidus-liquidus interval, which gives them the best casting properties.
In the Al-Si binary alloy, the solidus temperature is constant and amounts to 577 °C. As the silicon content increases, the liquidus temperature decreases from a maximum value for pure aluminum of 660 °C to coincide with the solidus temperature of 577 °C at a silicon content of 12.6%.
Among other alloying elements of aluminum, magnesium lowers the melting point the most: the eutectic temperature of 450 ° C is achieved with a magnesium content of 18.9%. Copper gives a eutectic temperature of 548 °C, and manganese - only 658 °C! Most alloys are not double, but triple and even quadruple. Therefore, with the combined influence of several alloying elements, the solidus temperature—the beginning of melting or the end of solidification—can be even lower.
Annealing hold and post-annealing cooling
When annealing, it is important to ensure that the specified temperature is achieved in all parts of the charge and at all points in each product. Therefore, a holding time at the annealing temperature of at least 1 hour is usually prescribed. The maximum annealing temperature is moderately critical: it is recommended not to exceed a temperature of 415 °C due to possible oxidation and grain growth. The heating rate can be critical, for example with alloy 3003, which typically requires rapid heating to prevent grain growth. Relatively slow cooling in still air or a furnace is recommended for all alloys to minimize warping.
Typical full annealing parameters for some aluminum alloys are presented below.
Process steps
- Recrystallization is a process in which deformed grains are replaced by a new set of undeformed grains, which form and grow until the original grains are completely consumed.
- Recrystallization annealing is an annealing process applied to a cold-worked metal to produce nucleation and growth of new grains without a change in phase. This heat treatment eliminates the effects of severe plastic deformation on high shaped cold stamped parts. Annealing is effective when applied to hardened or cold-rolled steels, which recrystallize the structure to form new ferrite grains.
- Recrystallization is usually accompanied by a decrease in the strength and hardness of the material and a simultaneous increase in ductility.
- Thus, the process may be introduced as an intentional metal processing step, or it may be an unwanted by-product of another processing step.
- The most important industrial applications are the softening of previously cold-worked metals that have lost their ductility and the control of grain structure in the final product.
- Recrystallization is defined as the process by which grains of a crystalline structure acquire a new structure or a new crystalline form.
- A precise definition of recrystallization is difficult to formulate because the process is closely related to several other processes, particularly grain extraction and growth.
- In some cases, it is difficult to pinpoint the point at which one process begins and another ends.
- "Formation of new grain structure in a deformed material through the formation and migration of high-angle grain boundaries caused by accumulated strain energy."
- The rate of microscopic mechanisms that control the nucleation and growth of recrystallized grains depends on the annealing temperature.
Full annealing parameters to remove strain hardening
Aluminum alloys
1060, 1100, 1350 3003, 3004, 3105 5005, 5050, 5052, 5083, 5086, 5154, 5182, 5254, 5454, 5456, 5457, 5652 7005 Also used for heat-hardening materials melts, if the purpose of annealing is only to remove strain hardening or partial annealing.
Annealing temperature
345 °C
Duration of exposure at annealing temperature
About 1 hour. The duration of stay in the furnace should be no longer than necessary to bring all parts of the charge to the annealing temperature.
Cooling after annealing
The cooling rate after annealing is not important.