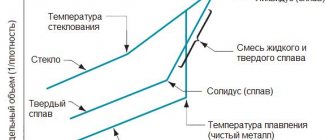
Aluminium oxide
| Aluminium oxide | |
| Are common | |
| Abbreviations | Corundum |
| Chemical formula | Al2O3 |
| Physical properties | |
| Condition (standard condition) | crystalline |
| Molar mass | 101.96 g/mol |
| Density | 3.99 g/cm³ |
| Thermal properties | |
| Melting temperature | 2044 °C |
| Boiling temperature | 2980 [1] °C |
| Enthalpy of formation (st. conv.) | −1675.7 kJ/mol |
| Classification | |
| Reg. CAS number | 1344-28-1 |
Aluminum oxide
Al2O3 - common in nature as alumina, a non-stoichiometric mixture of oxides of aluminum, potassium, sodium, magnesium, etc.
Thermophysical properties of metal oxides
The table shows the thermophysical properties of metal oxides (sintered oxides) at various temperatures.
The properties of the following dense sintered oxides are given: aluminum and magnesium oxides Al2O3, MgO, calcium oxide CaO, silicon oxide SiO2, nickel oxide NiO, titanium oxide TiO, zirconium oxide ZrO2, uranium oxide UO2, thorium oxide ThO2, plutonium oxide PuO2 Thermal conductivity of sintered oxides in the table is indicated at temperatures from 127 to 1727 °C depending on porosity. The coefficient of linear thermal expansion (CTE) is given at temperatures from 300 to 400 K. The density of metal oxides is given at room temperature.
The thermal conductivity of sintered metal oxides depends on the purity and crystalline structure of the original powders, the method and degree of pressing and sintering modes. The thermal conductivity of powdered oxides depends on density, grain size and humidity; for any powdered metal oxides (not sintered), the thermal conductivity is in the range of 0.1...1.1 W/(m deg).
The table shows the following properties of metal oxides :
- melting point, K;
- coefficient of linear thermal expansion (CTE), 1/deg;
- density, kg/m3;
- porosity, %;
- thermal conductivity coefficient, W/(m deg).
Aluminum. Chemistry of aluminum and its compounds
Binary aluminum compounds
Aluminum
Position in the periodic table of chemical elements
Aluminum is located in the main subgroup of group III (or in group 13 in the modern form of PSHE) and in the third period of the periodic table of chemical elements of D.I. Mendeleev.
Electronic structure of aluminum and properties
Electronic configuration of aluminum in the ground state :
+13Al 1s 2 2s 2 2p 6 3s 2 3p 1 1s
2s 2p 3s 3p
Electronic configuration of aluminum in excited state :
+13Al * 1s 2 2s 2 2p 6 3s 1 3p 2 1s
2s 2p 3s 3p
Thermal conductivity of dense sintered metal oxides
The table shows the thermal conductivity values of dense sintered metal oxides (porosity is zero) depending on temperature. Thermal conductivity is given for the following metal oxides: aluminum oxide Al2O3, beryllium oxide BeO, calcium oxide CaO, silicon oxide SiO2, magnesium oxide MgO, nickel oxide NiO, titanium oxide TiO2, zirconium oxide ZrO2. The thermal conductivity of metal oxides is given at temperatures from 100 to 2000 K.
It can be seen that, in general, the thermal conductivity of the oxides decreases with increasing temperature. The table also shows the density of metal oxides (oxide ceramics) at room temperature.
Aluminum
Aluminum is a chemical element of group III of the periodic table of Mendeleev (atomic number 13, atomic mass 26.98154). In most compounds, aluminum is trivalent, but at high temperatures it can also exhibit the +1 oxidation state. Of the compounds of this metal, the most important is Al2O3 oxide.
Aluminum is a silvery-white metal, lightweight (density 2.7 g/cm3), ductile, a good conductor of electricity and heat, melting point 660 °C. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it becomes covered with a protective oxide film - aluminum oxide) and reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide.
- Aluminum dissolves even in dilute hydrochloric and sulfuric acids, especially when heated. But aluminum does not dissolve in highly diluted and concentrated cold nitric acid. When aqueous solutions of alkalis act on aluminum, the oxide layer dissolves, and aluminates are formed - salts containing aluminum as part of the anion:
- Al2O3 + 2NaOH + 3H2O = 2Na[Al(OH)4] .
- Aluminum, devoid of a protective film, interacts with water, displacing hydrogen from it:
- 2Al + 6H2O = 2Al(OH)3 + 3H2
- The resulting aluminum hydroxide reacts with excess alkali, forming hydroxoaluminate:
- Al(OH)3 + NaOH = Na[Al(OH)4].
- The overall equation for the dissolution of aluminum in an aqueous alkali solution has the following form:
- 2Al + 2NaOH +6H2O = 2Na[Al(OH)4] + 3H2.
- Aluminum also actively interacts with halogens. Aluminum hydroxide Al(OH)3 is a white, translucent, gelatinous substance.
- The earth's crust contains 8.8% aluminum. It is the third most abundant element in nature after oxygen and silicon and the first among metals. It is part of clays, feldspars, and mica. Several hundred Al minerals are known (aluminosilicates, bauxites, alunites, and others). The most important aluminum mineral, bauxite, contains 28-60% alumina - aluminum oxide Al2O3.
- Density - 2.7 * 10 3 kg/m 3;
- Specific gravity - 2.7 g/cm3;
- Specific heat capacity at 20°C - 0.21 cal/deg;
- Melting point - 658.7°C;
- Specific heat capacity of melting - 76.8 cal/deg;
- Boiling point - 2000°C;
- Relative volume change during melting (ΔV/V) — 6,6%;
- Linear expansion coefficient (at a temperature of about 20°C) : - 22.9 * 10 6 (1/deg);
- Thermal conductivity coefficient of aluminum is 180 kcal/m*hour*deg;
Aluminum in its pure form was first obtained by the Danish physicist H. Oersted in 1825, although it is the most common metal in nature. Aluminum production is carried out by electrolysis of alumina Al2O3 in molten cryolite NaAlF4 at a temperature of 950 °C. Aluminum is used in aviation, construction, mainly in the form of aluminum alloys with other metals, electrical engineering (a substitute for copper in the manufacture of cables, etc.), food industry (foil), metallurgy (alloying additive), aluminothermy, etc.
Aluminum density, specific gravity and other characteristics.
Aluminum elastic modulus and Poisson's ratio
| Name of material | Young's modulus, kg/mm 2 | Shear modulus, kg/mm 2 | Poisson's ratio |
| Aluminum bronze, casting | 10500 | 4200 | — |
| Aluminum wire drawn | 7000 | — | — |
| Rolled aluminum | 6900 | 2600-2700 | 0,32-0,36 |
Reflection of light by aluminum
The numbers given in the table show what percentage of light incident perpendicular to the surface is reflected from it.
| Wave name | Wavelength | Light reflection, % |
| Ultraviolet | 1880 2000 2510 3050 3570 | 25 31 53 64 70 |
| Visible | 5000 6000 7000 | — — — |
| Infrared | 8000 10000 50000 100000 | — 74 94 97 |
ALUMINUM OXIDE Al2O3
Aluminum oxide Al2O3 , also called alumina, occurs in nature in crystalline form, forming the mineral corundum. Corundum has very high hardness. Its transparent crystals, colored red or blue, represent the precious stones ruby and sapphire. Currently, rubies are produced artificially by alloying with alumina in an electric furnace. They are used not so much for decoration as for technical purposes, for example, for the manufacture of parts for precision instruments, watch stones, etc. Ruby crystals containing a small admixture of Cr2O3 are used as quantum generators - lasers that create a directed beam of monochromatic radiation.
Corundum and its fine-grained variety containing a large amount of impurities - emery, are used as abrasive materials.
ALUMINUM PRODUCTION
The main raw material for aluminum production is bauxite containing 32-60% alumina Al2O3. The most important aluminum ores also include alunite and nepheline. Russia has significant reserves of aluminum ore. In addition to bauxite, large deposits of which are located in the Urals and Bashkiria, a rich source of aluminum is nepheline, mined on the Kola Peninsula. A lot of aluminum is also found in deposits in Siberia.
Aluminum is produced from aluminum oxide Al2O3 by the electrolytic method. The aluminum oxide used for this must be sufficiently pure, since impurities are difficult to remove from smelted aluminum. Purified Al2O3 is obtained by processing natural bauxite.
Aluminum oxide (Al2O3) has an exceptional set of properties:
- high hardness;
- good thermal conductivity;
- excellent corrosion resistance;
- low density;
- maintaining strength over a wide temperature range;
- electrical insulating properties;
- low cost relative to other ceramic materials.
These combinations make the material indispensable in the manufacture of corrosion-resistant, wear-resistant, electrical insulating and heat-resistant products for a wide variety of industries.
Main Applications:
- bearings for pumps and compressors;
- mechanical seal rings;
- ceramic plungers;
- elements of valves and shut-off valves;
- lining for protection against wear and corrosion;
- grinding bodies;
- lining for cyclones and calciners for cement plants;
- nozzles;
- crucibles, boats;
- thermocouple covers and tubes, rods;
- burners;
- electrical ceramics;
- products for the glass industry;
- fittings and lining of furnaces;
- positioning and welding pins for the automotive industry;
- products for the paper-making and printing industries;
- dies, eyes, dies, thread guides;
- substrates, plates;
- products for the metallurgical industry;
- abrasive and building materials, tools, welding ceramics;
- products for chemistry and petrochemistry.
There are two main types of aluminum oxide:
- dense aluminum oxide (Al2O3)
- fireproof aluminum oxide (Al2O3)
Dense aluminum oxide (Al2O3)
This material is used for the manufacture of critical and mechanically loaded products, i.e. mainly as engineering and structural ceramics. The material has excellent corrosion-resistant, wear-resistant, electrical insulating and heat-resistant properties, in addition to resistance to thermal shock. If thermal shock is present during operation, it is recommended to use fire-resistant materials.
There are several modifications of dense aluminum oxide depending on the content of the main phase and impurities, which are distinguished by strength and chemical resistance (ALOX-AP, ALOX-HP).
Basic properties of the material
| Properties | Material grade | |
| ALOX-AP | ALOX-HP | |
| Al2O3 94-96% | Al2O3 99.7% | |
| Density, g/cm3 | 3,6-3,7 | 3,8-3,9 |
| Closed porosity, % | 0 | 0 |
| Hardness, GPa | 12-13 | 15-17 |
| Bending strength, MPa | 300-330 | 350-400 |
| Compressive strength, MPa | 2000-2200 | 2000-2400 |
| Thermal conductivity at 20-100°C, W/mK | 20-25 | 28-33 |
| Coefficient of linear thermal expansion at 20-1000°C, 10-6K-1 | 7,0-8,0 | 7,0-8,0 |
| Maximum operating temperature Oxidizing environment Reducing or inert environment | 1500 1500 | 1750 1750 |
You can get advice on any issue you are interested in from our specialists.