The most fusible metals
Melting is the process of transition from a solid to a liquid state. It occurs under the influence of heat, but also depends on a number of physical factors, such as pressure. An important role in how easily and hard a substance can be melted is also played by its composition, the size of the crystals in the lattice, and the strength of the bonds between the atoms.
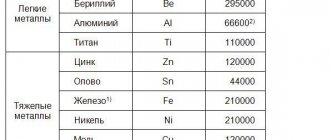
The melting point of metals varies greatly and can even have sub-zero values. It ranges from -39 to +3410 degrees Celsius. Molybdenum, tungsten, chromium, and titanium are the hardest to transform into liquid. For this process they need to be heated to a temperature of at least 2000 degrees.
The most fusible metals are gallium, mercury, lithium, tin, lead, zinc, indium, bismuth, and thallium. Read more about some of them below.
Application
Initially, refractory metals were used in the manufacture of capacitors and transistors for radio electronics. The number of their areas of application increased only by the middle of the 20th century. The industrial complex expanded to produce parts for machine tools, cars, airplanes and missiles.
Alloys that can withstand exposure to critical temperatures began to be used to make tableware. Refractory metals are used in the production of building and connecting materials. They are used to make parts for household appliances and electronics.
Tungsten is considered the most refractory. Its melting point of 3390 degrees exceeds other materials. However, we must not forget that if a tungsten part falls from a height, it will crack or break into separate parts.
Mercury
Useful in many areas, but poisonous metal was known even before our era. Mercury was used by ancient and medieval physicians to treat venereal and many other diseases; alchemists tried to make gold from it. Today it is used in electrical engineering, instrument making and organic chemistry.
Ruth is the most fusible metal on the planet. Under normal room conditions, it is always liquid, since its melting point is -39 degrees. Its vapors are very dangerous, so mercury is contained only in containers and special glass flasks. It acts like a poison on the body, poisoning it and disabling the nervous, immune, respiratory and digestive systems.
Gallium
The second most fusible metal is gallium. It becomes liquid at temperatures above 29.5 degrees Celsius, and can be softened simply by holding it in your hands for a while. Under normal conditions, gallium is very brittle, easily amenable to mechanical action, and has a light silver, somewhat bluish tint.
The metal is very dispersed in the earth's crust and is not found in the form of nuggets. In nature, it is found in various minerals, such as garnet, muscovite, tourmaline, chlorite, and feldspar. In addition, it is found in sea water. Gallium is used in high-frequency electronics, for the manufacture of mirrors and various alloys.
At what temperature does it melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
Different substances have different melting points. Theoretically, metals are divided into:
- Low-melting - temperatures up to 600 degrees Celsius are sufficient to obtain a liquid substance.
- Medium melting - requires a temperature of 600 to 1600 ⁰C.
- Refractory are metals that require temperatures above 1600 ⁰C to melt.
Melting iron
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Interesting: How to cook cast iron
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray - the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White - the temperature reaches 1350 degrees. It is poured into molds at 1450.
Important! The melting rates of a metal such as cast iron are 400 degrees lower compared to steel. This significantly reduces energy costs during processing.
Melting steel
Melting steel at a temperature of 1400 °C
Steel is an alloy of iron with an admixture of carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Reference! Steel melts at 1400 °C.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Indium
As a simple substance, indium is very light, malleable and soft, so much so that it even leaves a mark if it is passed over paper. It is also one of the most fusible metals, but is only affected by temperatures above 157°C. It boils at 2072 degrees.
Like gallium, indium does not form its own deposits, but is found in various ores. Due to its dispersion in nature, the metal is quite expensive. It is used in microelectronics, for the manufacture of low-melting alloys, solders, and liquid crystal screens for equipment.
Types and compositions of low-melting alloys
Low-melting alloys used in modern global industry:
| Alloy composition | Tmelt, | Density g/cm³ | Application area | Note | Other information |
| bismuth 76.5%, thallium 23.5% | 198 | T, P | Acid resistant | Eutectic alloy | |
| tin 89%, zinc 11% | 198 | T, P | |||
| bismuth 47.5%, thallium 52.5% | 188 | T | Eutectic alloy | ||
| bismuth 44.2%, lead 9.8%, thallium 48% | 186 | T | ∑? | Eutectic alloy | |
| tin 62%, lead 38% | 183 | 8,5 | T, P | ~POS 61 | |
| tin 64%, lead 36% | 181 | T, P | Eutectic alloy, ~POS 63 | ||
| sodium 70%, mercury 30% | 181 | T | Chemical act, Toxic. | ||
| cadmium 32%, tin 68% | 177 (178) | T, P | Toxic. | Eutectic alloy | |
| lead 32%, tin 68% | 177 | T, P | |||
| bismuth 12.8%, lead 49%, tin 38.2% | 172 | T, P | |||
| potassium 80%, thallium 20% | 165 | T | Chemical act | ||
| bismuth 13.3%, lead 46%, tin 40.1% | 165 | T, P | ∑? | ||
| bismuth 10.5%, lead 42%, tin 47.5% | 160 | T, P | |||
| bismuth 13.7%, lead 44.8%, tin 41.5% | 160 | T, P | Eutectic alloy | ||
| bismuth 16%, lead 36%, tin 48% | 155 | T, P | |||
| bismuth 18.1%, lead 36.2%, tin 45.7% | 151 | T, P | |||
| bismuth 25%, lead 50%, tin 25% | 149 | T, P | |||
| bismuth 62.5%, cadmium 37.5% | 149 | T, P | Toxic. | ||
| bismuth 19%, lead 38%, tin 43% | 148 | T, P | |||
| bismuth 50%, lead 50% | 145 | T, P | |||
| lead 32%, tin 50%, cadmium 18% | 145 | T, P | Toxic. | ||
| bismuth 60%, cadmium 40% | 144 | T, P | Toxic. | Eutectic alloy | |
| lead 42%, tin 37% | 143 | T, P | ∑? | ||
| cadmium 18.2%, lead 30.6%, tin 51.2% | 142 | 8,8 | T, P | Toxic. | ~POSK 50-18 |
| bismuth 57%, thallium 43% | 139 | T | Eutectic alloy | ||
| bismuth 57%, tin 43% | 139 | T, P | Eutectic alloy | ||
| mercury 70%, potassium 30% | 135 | T | Chemical act, Toxic. | ||
| potassium 90%, thallium 10% | 133 | T | Chemical act | ||
| bismuth 28.5%, lead 43%, tin 28.5% | 132 | T, P | |||
| bismuth 56%, tin 40%, zinc 4% | 130 | T, P | Eutectic alloy | ||
| bismuth 43%, lead 43%, tin 13% | 128 | T, P | ∑? | ||
| bismuth 27.2%, lead 44.5%, tin 33.3% | 127 | T, P | ∑? | ||
| bismuth 56.5%, lead 43.5% | 125 | T, P | Eutectic alloy | ||
| tin 52%, indium 48% | 125 | P | ~POIN 52 | ||
| bismuth 33.4%, lead 33.3%, tin 33.3% | 123 | T, P | ~POSV 33 | ||
| bismuth 36.5%, lead 36.5%, tin 27% | 117 | T, P | |||
| bismuth 40%, lead 40%, tin 20% | 113 | T, P | Bismuth Alloy | ||
| bismuth 42.1%, lead 42.1%, tin 15.8% | 108 | T, P | |||
| bismuth 48%, lead 28.5%, tin 14.5%, mercury 9% | 105 | T | |||
| bismuth 53%, tin 26%, cadmium 21% | 103 | T, P | Toxic. | ||
| bismuth 50%, tin 25%, cadmium 25% | 95 | T, P, M | Toxic. | ||
| bismuth 49.9%, lead 43.4%, cadmium 6.7% | 95 | T, P, M | Toxic. | ||
| bismuth 50%, lead 31.2%, tin 18.8% | 97 | T, P, M | Newton's alloy | ||
| bismuth 50%, lead 25–28%, tin 22–25% | 94–98 | T, P, M | Alloy Rose | ||
| bismuth 52.5%, lead 32.0%, tin 15.5% | 95 | T, P, M | Eutectic alloy | ||
| bismuth 51.6%, cadmium 8.1%, lead 40.3% | 91 | T, P, M | Toxic. | ||
| bismuth 55.2%, lead 33.3%, thallium 11.5% | 91 | T | Eutectic alloy | ||
| sodium 50%, mercury 50% | 90 | T | Chemical act, Toxic. | ||
| sodium 90%, mercury 10% | 90 | T | Chemical act, Toxic. | ||
| bismuth 53.2%, cadmium 7.1%, lead 39.7% | 89,5 | T, P, M | Toxic. | ||
| sodium 96.7%, gold 3.3% | 80 | T | Chemical act. | Eutectic alloy | |
| sodium 80%, mercury 20% | 80 | T | Chemical act, Toxic. | ||
| bismuth 35.3%, cadmium 9.5%, lead 35.1%, tin 20.1% | 80 | T, P, M | Toxic. | ||
| bismuth 58%, indium 17%, tin 25% | 79 | T, P, M | Eutectic alloy . Fields' alloy (English)Russian.. | ||
| bismuth 50%, lead 34.5%, tin 9.3%, cadmium 6.2% | 77 | T, P, M | Toxic. | ||
| bismuth 50%, lead 34.4%, tin 9.4%, cadmium 6.2% | 76,5 | T, P, M | Toxic. | ||
| bismuth 27.5%, cadmium 34.5%, lead 27.5%, tin 10.5% | 75 | T, P, M | Toxic. | ||
| bismuth 33.7%, indium 65.3% | 72 | T, P, M | ∑? | Eutectic alloy | |
| bismuth 38.4%, lead 30.8%, tin 15.4%, cadmium 15.4% | 71 | T, P, M | Toxic. | ||
| bismuth 49.5%, lead 27.27%, tin 13.13%, cadmium 10.1% | 70 | T, P, M | Toxic. | Eutectic alloy | |
| bismuth 50%, lead 26.3%, tin 13.3%, cadmium 10% | 70 | T, P, M | Toxic. | ||
| sodium 70%, mercury 30% | 70 | T | Chemical act, Toxic. | ||
| bismuth 48.8%, lead 24.3%, tin 13.8%, cadmium 13.1% | 68,5 | T, P, M | Toxic. | ||
| bismuth 52.2%, lead 26%, tin 14.8%, cadmium 7% | 68,5 | T, P, M | Toxic. | ||
| bismuth 50.1%, lead 26.6%, tin 13.3%, cadmium 10% | 68 | T, P, M | Toxic. | Lipovitsa alloy | |
| bismuth 50%, lead 25%, tin 12.5%, cadmium 12.5% | 68 | T, P, M | Toxic. | Wood's alloy | |
| bismuth 50.4%, lead 25.1%, tin 14.3%, cadmium 10.2% | 67,5 | T, P, M | Toxic. | Wood's alloy | |
| bismuth 50.1%, lead 24.9%, tin 14.2%, cadmium 10.8% | 65,5 | T, P, M | Toxic. | Wood's alloy | |
| sodium 99%, thallium 1% | 64 | T | Chemical act | Eutectic alloy | |
| bismuth 50.0%, tin 12.5%, lead 25%, cadmium 12.5% | 60,5 | T, P, M, F | Toxic. | ||
| bismuth 53.5%, tin 19%, lead 17%, mercury 10.5% | 60 | T | toxic | ||
| sodium 60%, mercury 40% | 60 | T | Chemical act. Toxic. | ||
| bismuth 49.4%, indium 21%, lead 18%, tin 11.6% | 57 | T, P, M, F | Eutectic alloy | ||
| mercury 70%, sodium 30% | 55 | T | toxic, reacts with water. | ||
| bismuth 42%, lead 32%, mercury 20%, cadmium 6% | 50 | T | toxic | ||
| bismuth 36%, mercury 30%, lead 28%, cadmium 6% | 48 | T | toxic | ||
| bismuth 47.7%, indium 19.1%, tin 8.3%, cadmium 5.3%, lead 22.6% | 47 | T, P, M, F | Toxic. | Eutectic alloy | |
| sodium 50%, mercury 50% | 45 | T | Chemical act. | ||
| bismuth 40.2%, cadmium 8.1%, indium 17.8%, lead 22.2%, tin 10.7%, thallium 1% | 41,5 | T, P, M, F | Toxic. | ||
| gallium 95%, zinc 5% | 25 | 5,95 | T | ||
| sodium 85.2%, mercury 14.8% | 21,4 | T | Chemical act. | ||
| gallium 92%, tin 8% | 20 | T | |||
| gallium 82%, tin 12%, zinc 6% | 17 | 6,13 | T | ||
| gallium 76%, indium 24% | 16 | 6,235 | T | ||
| gallium 67%, indium 29%, zinc 4% | 13 | 6,355 | T | ||
| Gallium 67%, indium 20.5%, tin 12.5% | 10,6 | T | |||
| gallium 62%, indium 25%, tin 13% | 4,85 | 6,44 | T | ||
| gallium 61%, indium 25%, tin 13%, zinc 1% | 3 | 6,4 | T | Russian alloy | |
| rubidium 91.8%, sodium 8.2% | −4,5 | 1,485 | T | Chemical act. | |
| potassium 77.3%, sodium 22.7% | −12,5 | 0,882 | T, L, I | Chemical act. | Eutectic alloy NaK |
| cesium 93%, sodium 7% | −28 | 1,765 | T, I | Chemical act. | |
| cesium 94.5%, sodium 5.5% | −30 | 1,778 | T, I | Chemical act. | |
| mercury 97.2%, sodium 2.8% | −48,2 | 13,16 | T | React with water. | |
| mercury 91.44%, thallium 8.56% | −61 | 13,45 | T | The most fusible amalgam | |
| sodium 12%, potassium 47%, cesium 41% | −78 | 1,28 | T, I | React. with water. | Soviet alloy |
Note: Several different melting points for the same alloy are the result of different interpretations of data sources
. Designations:
- T - coolant
- P - solder
- M - model casting alloy
- F - for fire alarm sensors
- L - laboratory for absolutization of solvents
- I - working fluid of ion rocket engines
↕
Tin
Tin melts at temperatures above 231 degrees Celsius. It is a ductile and soft metal, light silver in color. It exists in four allotropic modifications, two of which appear only at high pressure.
Tin is fairly dispersed in nature, but can form its own minerals, such as stannine and cassiterite. It is used as a coating for metals to enhance their resistance to corrosion, as well as for the production of tin, foil, various alloys, tableware and parts for musical instruments.
Lithium
Lithium is the most fusible metal, becoming a liquid at a temperature of 180 degrees. It is soft, easily forged and machined. It belongs to the alkali metals, but is much less active than other members of the group. It reacts slowly with moist air, and remains practically stable in a dry atmosphere
The metal is found in spodumene, lepidolite, in deposits with tin, bismuth and tungsten, found in sea water and in stellar space objects. Lithium is often used for the manufacture of galvanic cells, batteries, as an oxidizer, and also in pyrotechnics. In alloys with cadmium, copper and aluminum it is used in space, military and aviation technology.
What does melting temperature depend on?
For different substances, the temperature at which the structure is completely reconstructed to the liquid state is different. If we take into account metals and alloys, then it is worth noting the following points:
- Metals are not often found in their pure form. The temperature directly depends on its composition. As an example, we will indicate tin, to which other substances (for example, silver) can be added. Impurities make the material more or less resistant to heat.
- There are alloys that, due to their chemical composition, can transform into a liquid state at temperatures above one hundred and fifty degrees. There are also alloys that can “hold” when heated to three thousand degrees and above. Taking into account the fact that when the crystal lattice changes, the physical and mechanical qualities change, and operating conditions can be determined by the heating temperature. It is worth noting that the melting point of a metal is an important property of a substance. An example of this is aviation equipment.
Interesting: Welds - defects and their elimination
Heat treatment, in most cases, almost does not change the resistance to heat. The only sure way to increase resistance to heat is to make changes to the chemical composition; this is why steel is alloyed.