Fundamentals of metallurgical processes in welding
General information and features of welding metallurgy
By its nature, welding is a metallurgical process. Welding metallurgy is characterized by those physical and chemical processes that occur in the welding zone. They are determined by the interaction of molten metal with welding fluxes, slags and gases, as well as cooling and crystallization of the weld metal and transformations of the base metal in the heat-affected zone. These processes occur at all stages of arc welding: during the period of melting of the electrode, the transition of a drop of liquid metal through the arc gap and in the weld pool itself. However, in contrast to general metallurgy, characteristic of steel-making units, the conditions for the occurrence of metallurgical processes during welding differ in a number of features that affect both the course of their development and the results obtained. These features are:
- Small volume of the weld pool and at the same time quite large relative amounts of reacting phases in it.
- High temperatures in various areas of the welding zone and high overheating of the melt in the bath.
- Movement of liquid metal, intensive mixing of molten products and their continuous renewal and exchange in the weld pool.
- High cooling and crystallization rates of the deposited metal.
Under these conditions, active interaction of the molten metal with the surrounding gas environment and fluxes heated to high temperatures is observed. Processes occur at high speed. However, due to the short-term existence of the melt and the constant renewal of the interacting phases, most often they do not reach complete completion and most reactions in the welding zone do not reach an equilibrium state. In addition, conditions are created that prevent the complete cleansing of the weld metal from various non-metallic inclusions, oxides and gases, which, due to the rapid solidification of the melt, do not have time to reach the surface of the weld pool and are removed into the slag. On the other hand, high rates of cooling and crystallization of the metal significantly affect the structure of the resulting welds, leading to a fine-grained structure, a decrease in chemical heterogeneity, and as a result, an increase in the properties of the cast weld metal.
The metallurgical processes that take place are associated with the occurrence of certain chemical reactions, which can result in oxidation or deoxidation of the weld metal, alloying it with certain elements, dissolution and release of gases in the weld, etc. Some of them lead to deterioration of the properties of the resulting compounds and are undesirable (for example , oxidation), others help improve the quality and properties of compounds and are often carried out deliberately, such as deoxidation. Therefore, in a particular case of assigning welding conditions, it is necessary to proceed from an analysis of the entire complex of physical and chemical processes, bearing in mind that their overall result should be the production of weld metal with certain properties and a certain chemical composition. This is determined not only by the composition of the filler and base metal, but also largely depends on the nature and intensity of the reactions occurring during the welding process.
The main processes occurring during arc welding
There are many processes occurring under arc welding conditions. Let's consider those that are common in all or most cases of welding.
Dissociation of gases and compounds.
Dissociation occurs when more complex components break down into atoms or constituent parts. This process is facilitated by the presence of high temperatures in the welding zone and the catalytic effect of the molten metal. During arc welding, the molecules of gases, both simple - oxygen, nitrogen, hydrogen, and complex - carbon dioxide CO2, water vapor H2O, etc., are first subject to dissociation. Dissociation of gases occurs according to reactions
Oxygen and hydrogen at arc temperatures almost completely dissociate into atoms, nitrogen dissociates to a lesser extent.
The dissociation of water vapor, depending on temperature, occurs according to the reactions
Consequently, depending on the reaction conditions, water vapor can oxidize or reduce the metal of the weld pool.
More complex compounds also undergo dissociation. Many electrode coatings and fluxes contain fluorspar CaF2. At high temperatures it decomposes according to the reaction
Fluorine atoms, when combined with electrons, turn into ions with low mobility. This leads to a decrease in the conductivity of the arc gap and a deterioration in the stability of the arc. But at the same time, fluorine atoms are able to bind hydrogen into HF molecules that do not dissolve in the bath metal, reducing the saturation of the weld metal with hydrogen.
Many electrode coatings contain carbonates, such as CaCO3. When decomposing at high temperatures, they release carbon dioxide, which in turn dissociates to form oxygen:
Being in an atomic state, gases become chemically active and, reacting with the metal, sharply deteriorate its quality.
Metal oxidation during welding.
The metal of the weld pool can be oxidized due to the oxygen contained in the gas environment and slag in the welding zone. In addition, oxidation can also occur due to oxides (scale, rust) located on the edges of parts and the surface of the electrode wire. When heated, the moisture present in the rust evaporates, the water molecules dissociate, and the resulting oxygen oxidizes the metal. When metal melts, scale turns into iron oxide, also releasing free oxygen. If the weld pool is not adequately protected, oxidation occurs due to atmospheric oxygen.
Oxygen with iron forms oxides: FeO (22.3% O2), Fe3O4 (27.6% O2), Fe2O3 (30.1% O2). At a high temperature of the welding arc due to atomic oxygen as a result of the reaction Fe + O
FeO forms a lower oxide, which, when the temperature decreases, can transform into other forms of higher oxides.
The greatest danger to the quality of the weld is the oxide FeO, which can dissolve in liquid metal. This oxide has a melting point lower than that of the base metal. Therefore, when the weld metal crystallizes, it hardens last. As a result, it is located in the form of layers along the grain boundaries, which causes a decrease in the plastic properties of the weld metal. The more oxygen in the weld is in the form of FeO, the more its mechanical properties deteriorate. Higher iron oxides do not dissolve in liquid metal and, if they do not have time to float to the surface of the weld pool, they remain in the weld metal in the form of slag inclusions.
Iron can also be oxidized due to oxygen contained in CO2 and water vapor H2O:
During the welding process, in addition to iron, other elements found in steel are also oxidized - carbon, silicon, manganese. During the transition of droplets of electrode metal in the arc, oxidation of the elements occurs as a result of their interaction with the atomic oxygen of the gaseous medium of the arc gap: C + O = CO, Mn + O = MnO, Si + 2O = SiO2.
In the weld pool, elements are oxidized when they interact with iron oxide:
Oxidation of these elements leads to a decrease in their content in the weld metal. In addition, the resulting oxides can remain in the weld in the form of various inclusions, which significantly reduce the mechanical properties of welded joints, especially the ductility and impact strength of the weld metal. An increased oxygen content also has a harmful effect on other properties - it reduces resistance to corrosion, increases the susceptibility to aging of the metal, and makes it cold-brittle and red-brittle. Therefore, one of the conditions for obtaining high-quality weld metal is to prevent its oxidation in the first place by creating various protective environments.
Deoxidation of metal during welding.
The protective measures used during welding do not always ensure the absence of oxidation of the molten metal. Therefore, it needs to be deoxidized. Deoxidation is the process of reducing iron from its oxide and converting oxygen into the form of insoluble compounds, followed by their removal into slag. Oxidation and deoxidation, in essence, represent two directions of the same chemical process. In the general case, the deoxidation reaction has the form FeO + Me = Fe + MeO, where Me is the deoxidizer.
A deoxidizing agent is an element that, under welding conditions, has a greater affinity for oxygen than iron. Silicon, manganese, titanium, aluminum, and carbon are used as deoxidizers. Deoxidizers are introduced into the weld pool through electrode wire, electrode coatings and fluxes. Below are the most typical deoxidation reactions.
Manganese deoxidation:
Fe + Mn= Fe + MnO
Manganese oxide is slightly soluble in iron, but it itself dissolves iron oxide FeO well, carrying it along with it into the slag.
Silicon deoxidation:
2FeO + Si= 2Fe + SiO2.
Titanium deoxidation: 2FeO + Ti = 2Fe + TiO. Titanium is an energetic deoxidizer, which produces low-melting titanates of manganese and iron:
Silicon oxide is poorly soluble in iron and floats into the slag. Deoxidation by silicon is accompanied by reactions of the formation of more fusible complex silicates of manganese, silicon and iron, which better transform into slag:
Manganese, silicon and titanium are introduced into the weld pool through the electrode wire, alloying it through the electrode coating or flux, introducing the corresponding ferroalloys.
Carbon deoxidation:
FeO + C = Fe + CO.
The resulting carbon monoxide is released into the atmosphere in a gaseous state, causing strong boiling of the weld pool and forming pores in the weld. To obtain tight welds, the deoxidation reaction with carbon should be “suppressed” by introducing other deoxidizers, such as silicon, into the weld pool.
Interaction with nitrogen.
Nitrogen in the air, entering the arc column, heats up and partially dissociates. In the atomic state, nitrogen dissolves in liquid metal. During the cooling process, nitrogen falls out of solution and interacts with the metal, forming a number of compounds - nitrides Fe2N, Fe4N. Atomic nitrogen can also combine with oxygen, forming nitrogen oxide NO, which, dissolving in drops of electrode metal, goes into the weld pool. The nitrogen content in the weld metal adversely affects its mechanical properties, especially ductility. In addition, saturation of the metal with nitrogen promotes the formation of gas pores. Nitrogen reduction is carried out to protect the molten metal from air or to introduce chemical elements into it that remove nitrogen in the form of non-metallic inclusions.
Interaction with hydrogen.
Hydrogen can enter the welding zone from moisture in the electrode coating or flux, rust on the surface of the welding wire and workpiece, or from the air. Atomic hydrogen is highly soluble in liquid metal, and the solubility increases with increasing heating temperature. An important regularity in the behavior of gases is the abrupt change in their solubility in a metal during its phase changes and especially during the transition from a liquid to a solid state (Fig. 1).
When the weld pool cools and crystallizes, the hydrogen released does not have time to be completely removed from the weld metal. This leads to the formation of gas pores in it. In addition, hydrogen atoms, diffusing into existing cavities and discontinuities in the solidifying metal, lead to an increase in pressure in them, the development of internal stresses in the metal and the formation of microcracks. Reducing gas saturation of seams is carried out due to high-quality protection of the molten metal during welding by cleaning and calcining the welded and welding materials.
Reactions with sulfur and phosphorus.
Sulfur is a harmful impurity in steels. It enters the weld pool from the base metal, welding wire and sometimes from the electrode coating or flux. In metal, sulfur can be in the form of compounds - sulfides. Iron sulfide FeS, which is highly soluble in iron, is especially harmful. The presence of sulfur in the weld metal reduces its mechanical properties and greatly increases the tendency to crack. Therefore, desulfurization, the purification of metal from sulfur, aims to reduce the total sulfur content in the weld and especially FeS. Desulfurization is carried out by introducing into the weld pool elements that have a greater affinity for sulfur than iron. The resulting sulfide of the element should dissolve poorly in the metal and well in the slag. Such an element is manganese, which has a high affinity for sulfur. Manganese sulfide does not dissolve in the metal, has a low density and easily floats into the slag of the weld pool. The process follows reactions
The same effect is achieved by introducing calcium according to the reaction FeS + CaO = FeO + CaS.
Calcium oxide is obtained by decomposing CaCO3 marble in an arc.
Reactions with phosphorus.
Phosphorus is also a harmful impurity in steels. The ways it gets into the seam are the same as for sulfur. In the metal, phosphorus is found in the form of compounds - iron phosphides with a melting point much lower than that of iron (1170 ° C):
Then the binding of phosphorus oxide occurs according to the reactions
Phosphorus in the weld metal is located along the grain boundaries in the form of a fusible layer and leads to strong heterogeneity of the metal, grain growth and a decrease in ductility, especially at low temperatures, causing cold brittleness of the metal. Phosphorus is removed by oxidation and subsequent binding into a strong compound, which is removed into slag:
The resulting compounds are discharged into slag.
Features of metallurgical processes for various types of welding
Submerged arc welding.
In automatic and mechanized submerged arc welding, the welding arc burns in a flux gas bubble filled with hot gases of the arc column and flux vapor. The conditions for metallurgical processes differ in a number of features:
- more effective protection of the weld pool from oxygen and nitrogen in the air (in submerged arc welds, the nitrogen content does not exceed 0.008%);
- the volume of the weld pool is larger than with manual arc welding, and the time it remains in the molten state is longer, which contributes to a more complete occurrence of chemical reactions between the liquid metal and the slag;
- a more stable relationship between the welding mode and the chemical composition of the molten metal, which makes it possible to obtain the specified composition of the weld metal with sufficient accuracy and stability.
One of the features of metallurgical processes during submerged arc welding is alloying the weld with manganese and silicon by reducing them from the oxides MnO and SiO2 contained in the flux. In the high-temperature welding zone, reduction reactions occur:
The resulting FeO oxide partially floats into the slag and partially dissolves in the liquid metal. Manganese and silicon are completely dissolved in the metal.
In the tail part of the weld pool in the zone of low temperatures, deoxidation reactions occur due to Mn and Si, which have a greater affinity for oxygen under these conditions than iron:
The resulting oxides combine with each other to form complex low-melting silicates of manganese and iron, which easily float into the slag.
Gas shielded arc welding.
Of the active protective gases, carbon dioxide CO2 is the most widely used. The peculiarity of metallurgical processes in this case is due to its strong oxidizing effect. The gas environment in an arc burning in CO2 is more oxidizing in nature (33% O2) than when it burns in air (21% O2). Therefore, strong oxidation of the weld pool is observed according to the reaction Fe + CO2 = FeO + CO.
At the same time, carbon dioxide dissociates. Atomic oxygen also oxidizes iron and other impurities in the weld pool: silicon, manganese, carbon, etc. These reactions occur both during the transition of electrode metal droplets in the arc and on the surface of the pool itself. To control the oxidation reaction, as well as to replenish the loss of elements, electrode wires with a high content of manganese and silicon (Sv-08GS, Sv-08G2S, etc.) are used. When using these wires in the zone of lowering the temperature in the weld pool, deoxidation reactions occur:
The resulting oxides of manganese and silicon float to the surface of the weld pool.
Inert protective gases (argon, helium) do not dissolve in the molten metal and do not form chemical compounds in the bath.
Oxidation of the weld pool is facilitated by impurities in the shielding gas in the form of free oxygen and water vapor. In this case, mainly carbon is oxidized to form gaseous oxide CO. To suppress the carbon oxidation reaction, a sufficient amount of silicon and manganese deoxidizers must be present in the weld pool. For this purpose, when welding carbon steels, the same electrode wires are used as when welding in carbon dioxide, with a higher content of deoxidizing agents.
Crystallization of the weld pool
The weld seam in arc welding is formed by crystallization of the molten metal of the weld pool. Crystallization is the process of formation of metal crystals from a melt during its transition from liquid to solid state. The metal crystals formed in this process are usually called crystallites.
The weld pool can be conditionally divided into two areas: front (head) and rear (tail). In the front part the arc burns and the metal is heated and melted, and in the tail part the melt cools and crystallizes. In the process of weld formation, primary and secondary crystallization are distinguished. Primary crystallization is the direct transition of a metal from a liquid to a solid state with the formation of primary crystallites (grains). It occurs at high rates of cooling and solidification. Heat is transferred to the base metal surrounding the weld pool. In general, the crystallization process consists of two stages: the formation of crystallization centers (nuclei) and the growth of crystals from these centers. During the primary crystallization of the weld metal, the surfaces of the melted grains of the base metal surrounding the weld pool are the centers of crystallization. In this case, common grains appear between the base metal and the weld metal. The conventional interface between the grains of the base metal and the crystallites of the weld is called the fusion zone during welding.
During the solidification process, new crystallization centers may appear in the melt - refractory impurity particles, grain fragments, etc.
In multilayer welding, the crystallization centers are the surfaces of the grown crystallites of the previous layer. The growth of crystallites occurs as a result of the attachment of individual atoms from the surrounding melt to their surface. Depending on the shape and location of the crystallites in the structure of the solidified weld metal, a columnar and granular structure is distinguished. With a columnar structure, the crystallites have a certain orientation - they are elongated in one direction, opposite to the direction of heat removal. In turn, columnar crystallites themselves can have a cellular, cellular-dendritic or dendritic structure. With a cellular structure, a columnar crystallite grows from the surface of a common center in the form of a pack of thin crystals located within the same grain and oriented in the same direction. This is observed at a high rate of heat removal. As the rate of heat removal decreases, the nature of its structure changes, moving to a cellular-dendritic and dendritic form. With a dendritic structure in a crystallite, in addition to first-order axes, second- and third-order axes also develop.
With a granular structure of the weld metal, the crystallites do not have a specific orientation, but are shaped like polyhedra. This structure is usually characteristic of the base metal, and can also occur in welds with a large volume of the weld pool and at low cooling rates of the melt. Therefore, the crystallized weld metal in most cases has a columnar structure. Depending on the welding conditions, the sizes of columnar crystallites vary within wide limits. In arc welding, their cross-sectional size is usually on the order of 0.3 - 3.0 mm.
The primary crystallization of the weld pool metal is intermittent. After the start of crystallization, after some time there is a delay in the growth of crystallites due to the release of latent heat of melting of the metal. As heat is removed, the growth process accelerates again until the next delay. This is repeated until the entire bath is completely hardened. As a result, welds have a characteristic layered structure (Fig. 2). The thickness of the crystallization layers is measured in the range from tenths to several millimeters, depending on the volume of the bath and heat removal conditions. The columnar crystallites of each subsequent layer are a continuation of the crystallites of the previous layer. As a result, the resulting crystallites seem to grow from layer to layer.
The nature of the resulting structure and the arrangement of crystallites in the weld metal are largely determined by the shape of the weld pool and the pattern of its crystallization. Crystallites grow perpendicular to the fusion boundary in the direction opposite to heat removal. During crystallization of a weld pool with a narrow, deep penetration, crystallites grow from opposite walls towards each other. In this case, various types of impurities accumulate in front of the crystallization front. As a result, along the axis of the weld, at the junction of the tops of crystallites growing from opposite sides of the bath, a weakening area is formed, in which various inclusions can be located (Fig. 3, a). When a wide weld pool solidifies with little penetration, the crystallization pattern is significantly different - the crystallites touch not at their tops, but at their side faces, and the impurities that concentrate in front of the crystallization front are displaced onto the surface of the weld in the form of slag. Such seams are more resistant to crack formation (Fig. 3, b).
During the crystallization process, the composition of the liquid metal in the bath continuously changes. Therefore, simultaneously with crystallization, diffusion processes develop in it, tending to a homogeneous composition of the metal both inside the crystallites and between the solidified crystallites and the remaining liquid melt. However, due to the difference in the growth rates of crystallites and diffusion processes, which are slower, complete equalization of the composition does not occur.
Rice. 3. Scheme of bark crystallization depending on the shape of the weld pool: a - narrow weld pool with deep penetration, b - wide weld pool
This leads to uneven distribution of alloy elements of the welded seam - chemical heterogeneity of the weld metal. A distinction is made between macroscopic and microscopic heterogeneity. The first type is characterized by uneven composition in certain areas of the metal along the cross-section of the weld (zonal segregation). With microscopic heterogeneity, uneven composition of the metal is observed within individual crystallites (microscopic segregation). Due to segregation, chemical heterogeneity of the weld metal is created. Intradendritic heterogeneity usually develops predominantly in welds. The intensity of manifestation of segregation processes depends on the welding conditions. The faster the metal solidifies, the less segregation occurs. The type and degree of chemical heterogeneity have a significant impact on the properties of the weld metal, its resistance to cracking, etc.
The study and analysis of the structure of the weld metal is carried out by identifying its crystalline structure on specially prepared sections of transverse and longitudinal sections. At the same time, a distinction is made between the concepts “macrostructure” and “microstructure”. Macrostructure is the structure of the weld metal, revealed by inspection with the naked eye or at low magnifications using magnifying glasses or binocular microscopes. In this case, it is possible to identify the general nature of the metal structure (columnar, granular), the shape of the penetration, the presence of defects (pores, cracks, inclusions, etc.). The microstructure of the weld metal characterizes its fine structure, revealed on thin sections using metallographic microscopes with a high degree of magnification (structure of crystallites, the presence of intradendritic segregation, microdefects).
Formation of cracks and gas pores in the weld metal
During the crystallization of the weld pool, cracks may form in the weld metal. According to their location relative to the axis of the seam, they can be longitudinal and transverse, depending on the size - micro- and macroscopic (the first of them are detected using a microscope, and the second - with the naked eye); Depending on the temperatures at which they form, cracks are divided into two groups: hot (high temperature) and cold (low temperature). The mechanism of their occurrence is different.
Hot cracks are brittle intercrystalline fractures of the weld metal and heat-affected zone that occur during crystallization in the solid-liquid state, as well as at high temperatures in the solid state. Cracks, as a rule, are located along the boundaries of crystallites and cause intercrystalline fracture. This is explained by the fact that when the weld metal solidifies during the process of primary crystallization, liquid layers with a low melting point are located between the crystallites. If the tensile internal stresses arising in the metal at this time (due to linear shrinkage during cooling) are sufficiently large, then destruction will occur along these layers with the formation of a crack. If the process of complete solidification of the melt ends before the appearance of large tensile stresses, then hot cracks do not form. The formation of hot cracks is facilitated by the content of impurities in the weld metal - sulfur, phosphorus, etc. Thus, sulfur forms a low-melting iron sulfide FeS, which during crystallization is located along the grain boundaries and increases the likelihood of crack formation. The formation of cracks is also affected by the shape and crystallization pattern of the weld pool. Narrow welds with deep penetration are more prone to cracking than wide welds with little penetration. To reduce the risk of hot cracks, the following measures are used: use welding materials with a minimum content of sulfur, carbon, phosphorus; increase the content of manganese in the weld metal, which binds sulfur into a more refractory compound - manganese sulfide; the bath melt is refined (cleaned) from sulfur by introducing components containing calcium.
Cold cracks
in the metal structure they are located both along the boundaries and along the body of grains. Therefore, they represent intracrystalline fractures. Cold cracks in welded joints form at temperatures of 200 - 300°C. Most often they form in seams when welding hardening steels. The tendency of the metal to form cold cracks is influenced by the increased content of carbon and elements that facilitate hardening, the presence of hydrogen in the weld, phosphorus contamination, rapid cooling and the presence of internal stresses in the welds. In order to reduce the tendency of the metal to form cold cracks, the following measures are used: use materials with a minimum phosphorus content, reduce the saturation of the weld pool with hydrogen and nitrogen, and take measures to reduce internal stresses.
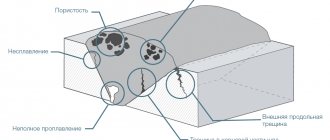
Pores in welds arise during the primary crystallization of the metal of the weld pool as a result of the release of gases. Pores are cavities in the seams, filled with gas, having spherical, elongated or more complex shapes. Pores can be located along the axis of the weld, its cross-section, or near the fusion boundary. They can be hidden in the metal or come to the surface, arranged in chains, separate groups or singly, and can be microscopic and large (up to 4 - 6 mm in diameter). Pores during welding mainly arise due to hydrogen, nitrogen and carbon monoxide gases formed as a result of chemical reactions with the release of gas products, the release of gases due to their different solubility in liquid and solid metal, and the capture of gas from the environment during crystallization of the weld pool.
To reduce porosity, it is necessary to carefully prepare the surface of the base and filler metals for welding (removal of rust, oil, moisture, calcination, etc.), reliable protection of the welding zone from air, and the introduction of deoxidizing agents into the weld pool (from the base metal, welding wire, coating, flux), stable adherence to welding conditions.
Weld joint structure
The welded joint (Fig. 4) during fusion welding includes weld seam 1, formed as a result of crystallization of the weld pool, fusion zone 2 and heat-affected zone 3, which represents the part of the base metal that is directly accustomed to the weld seam and is subject to thermal effects during welding, causing changes in structure and properties.
The metal in any zone of the welded joint experiences heating and cooling. The change in metal temperature over time is called the thermal cycle of welding. The maximum heating temperature in different areas of the connection is different.
The weld is formed as a result of the melting of the base and electrode metals, and therefore, after solidification, it has the structure of cast metal with elongated columnar crystallites. In the heat-affected zone, the heating changes from the melting temperature at the boundary with the seam to room temperature. In this case, various structural and phase transformations can occur in the metal, leading to the appearance of sections of the metal that differ in structure.
When welding low-carbon steels, areas (Fig. 5) of incomplete melting, overheating, normalization, incomplete recrystallization, recrystallization and blue brittleness are noted.
The area of incomplete melting is adjacent directly to the weld and is transitional from the cast weld metal to the main one. In this area, a connection is formed and the fusion boundary passes. It represents a narrow region (0.1 - 0.4 mm) of the base metal, heated until the grains partially melted. The overheating area is an area of the base metal heated to temperatures of 1100 - 1450°C, and therefore its metal has a coarse-grained structure and reduced mechanical properties and is more noticeable the larger the grain and the wider the overheating zone. The normalization (recrystallization) section covers the area of the base metal heated to a temperature of 900 - 1100°C. The metal in this area has high mechanical properties, since when heated and cooled, a fine-grained structure is formed in this area as a result of recrystallization without overheating.
The area of incomplete recrystallization is heated within the temperature range of 725 - 900°C. Due to incomplete recrystallization caused by insufficient heating time and temperature, the metal structure consists of a mixture of small recrystallized grains and large grains that did not have time to recrystallize. Its properties are lower than those of the metal of the previous section.
The recrystallization area is observed during welding of steels subjected to cold deformation (rolling, forging, stamping). When heated to a temperature of 450 - 725°C, a recrystallization process develops in this area of the base metal, leading to grain growth, coarsening of the structure, and softening of the metal.
The area heated in the temperature range 200 - 450°C is transitional from the heat-affected zone to the base metal. Metal aging processes may occur in this area due to the precipitation of iron carbides and nitrides. The ductility and toughness of the metal decreases. The structure of this section is practically no different from the base metal. Thus, the welded joint is characterized by heterogeneity of properties. The width of the heat-affected zone depends on the thickness of the metal, the type and mode of welding. For example, with manual arc welding it is usually 5 - 6 mm.
Chemical processes in the weld pool. Harmful impurities and their removal from the weld pool.
⇐ PreviousPage 4 of 9Next ⇒
The main harmful impurities for the weld are oxygen, nitrogen, hydrogen, sulfur, and phosphorus.
Oxygen - worsens the mechanical and technological properties (ductility, workability), wear resistance of the weld metal, and can lead to the formation of pores. The source of oxygen is the surrounding air.
The oxygen content in the weld depends on the length of the arc, the strength of the welding current, and protection conditions. As the arc length and welding current increase (when the size decreases, but the number of droplets of electrode metal increases), the area of contact of the molten metal with air increases - the amount of oxygen entering the weld increases.
Nitrogen forms nitrides of iron (Fe4N, Fe2N), manganese and other elements, which are located in the weld in the form of needle-shaped inclusions, leading to the appearance of cracks in the welded structure at low temperatures (cold brittleness).
Nitrogen can enter the weld from the air, i.e. The nitrogen content in a weld depends on the same factors as oxygen.
Hydrogen entering the weld pool causes cracks and pores in the weld. Sources of hydrogen can be moisture in the electrode coating, rust and other contaminants on the edges. As the doping level increases, the susceptibility to hydrogen cracks increases.
Sulfur and phosphorus can get into the weld pool from the base metal, welding wire, electrode coating, and fluxes. Sulfur causes cracks to appear at high temperatures (“red brittleness”), and phosphorus causes cracks to appear at low temperatures (“blue brittleness”).
Reducing the content of harmful impurities in the weld is achieved by gas-slag protection of the molten metal - when the coating of the electrode or flux melts, a gas is formed surrounding the arc and slag is formed, enveloping drops of molten metal and the weld pool; the slag also slows down the cooling of the liquid metal.
In addition, to obtain a high-quality seam, special chemical processes in the weld pool:
1. Weld deoxidation - removal of oxygen from the weld due to the addition of special elements.
These elements must meet two requirements
: firstly, they must have a greater affinity for oxygen than iron, i.e. they must “take” oxygen from iron, reducing it from oxides; secondly, these new compounds must be insoluble in steel, i.e. come out of the seam into the slag.
The following elements (“deoxidizers”) meet these requirements:
o Carbon (C ) is an “automatic deoxidizer”, i.e. it is not specifically added to the weld pool - it is always present in the steel and enters the weld pool from the base metal, filler material (wire), electrode coating, and flux.
2FeO + C = 2Fe+ CO2
Carbon dioxide (CO2) must have time to escape from the weld into the slag before crystallization, otherwise there may be pores.
o Manganese (Mn) deoxidizes the weld and removes sulfur (S):
FeO + Mn = Fe + MnO
FeS + Mn = MnS + Fe
The compounds indicated by the arrows should go to the slag.
o Silicon (Si) is a stronger deoxidizing agent than manganese, but it is used together with manganese, because the SiO2 compound is viscous, and manganese gives them fluidity, i.e. promotes release into slag. Otherwise, weld defects – “slag (non-metallic) inclusions” – may occur.
o Titanium (Ti) – a strong deoxidizing agent
Ti + 2Fe = TiO2 + 2Fe
2. Alloying of the weld - introduction of special noble elements into the weld (Mn, Si, Cr, Ni, Mo, W, Ti, etc.), which improve the chemical composition and structure of the weld metal. Alloying elements can be introduced through filler materials (wire), electrode coatings, and flux.
3. Cleaning the seam from sulfur and phosphorus–
Sulfur forms FeS compounds - upon crystallization, low-melting compounds are obtained along the boundaries of metal grains, which at high temperatures, melting, form cracks; removal - manganese
FeS +Mn= MnS+ Fe
FeS + MnО=MnS+ FeО
Phosphorus reduces the mechanical properties of the metal, leading to blue brittleness (cracks at low temperatures)
Removal - in 2 stages:
– Oxidation 2Fe2P + 5FeO = P2O5 + 9Fe;
2Fe3P + 5FeO = P2O5 + 11Fe
– Binding into chemical compounds (oxides of Ca, Mg, Mn), insoluble in steel
3CaO + P2O5 = Ca3P2O8
4CaO + P2O5 = Ca4P2O9
Weldability of metals.
Weldability is the ability of metals to form a high-quality welded joint that meets operational requirements.
Methods for assessing weldability:
• Direct - welding of samples in various modes with subsequent testing of samples for tensile, bending, impact strength, corrosion resistance, etc.
• Indirect - based on carbon equivalent.
, (3)
Where; C – carbon content, %;
Mn, Cr, ... - content of alloying elements,%.
Table 1 - Weldability groups
| Weldability group | Sack | Welding conditions |
| I Good | Up to 0.25 | No limits |
| II Satisfactory | 0,25-0,35 | Only at an ambient temperature of at least 50C, metal thickness ˂ 20 mm and no wind |
| III Limited | 0,35-0,45 | With preliminary or accompanying heating up to 2500C |
| IV Bad | Over 0.45 | With preliminary and accompanying heating, heat treatment after welding |
Control questions:
1. List the main harmful impurities in welds, explain their effect on the properties of the weld.
2. What is a “deoxidizer”, what requirements must it meet?
3. List the elements used to deoxidize the weld pool, explain the features of their use.
4. How do sulfur and phosphorus affect the quality of the weld, where can they get into the weld, and how can they be removed from the weld pool?
5. Define the concept of “weldability of metals.” Explain the methods for assessing weldability, weldability groups.
⇐ Previous4Next ⇒
Recommended pages:
Processes occurring during metal welding
All types of fusion welding are complex processes, but in any of these types, processes that are similar in nature occur. There are three main processes that occur when welding metals: 1. Welding metals directly to each other, or with the help of an intermediate molten metal. 2. The effect of heat generated during welding on the base metal being welded. 3. Melting, metallurgical processing and subsequent crystallization of the metal that makes up the weld.