Appearance and characteristics of technical calcium carbide
The composition in question was first obtained in 1862. The procedure involved the separation of calcium from lime, resulting in a pale gray composition without the characteristics characteristic of metals. As a result of the experiment, carbide was obtained, which subsequently began to be actively used in the production of various products.
At the beginning of the 20th century, calcium carbide began to be used to produce acetylene in large volumes. That is why active research began to be conducted to identify more productive technology.
The technical characteristics of the material determine its wide distribution. The appearance of the substance is characterized by a light gray color; carbides are produced in the form of stone or powder.
Physical properties
When choosing almost any material, you should pay most attention to physical properties. For the one under consideration they are as follows:
- The compound has a crystalline structure.
- The melting point is 2300 °C. It is worth considering that such a figure is characteristic only of the pure composition. The addition of various impurities to the composition causes the melting point to drop significantly.
Pure Calcium Carbide
It is worth considering that calcium carbide in most cases is in a solid state. In addition, the color can vary from gray to brown. The physical properties of calcium carbide determine its widespread use in a wide variety of industries.
Chemical properties
Chemical properties are also important. They are also taken into account when using the material. The main characteristics include the following qualities:
- Calcium carbide is characterized by its ability to absorb moisture well. It is worth considering that such a procedure is manifested by a vivid chemical reaction associated with the decomposition of the substance.
- When working with the material in question, it is worth considering that the resulting dust has an irritating effect on the mucous organs. In addition, a similar reaction can occur when crystals or dust come into contact with the surface of the skin. That is why when working with the compound in question, you should use a respirator and some other protective equipment.
- Crystals actively react to the influence of other substances, often only when heated. This may result in the formation of calcium carbonate.
- In some cases, a crystalline substance is combined with nitrogen, resulting in calcium cyanamide.
- When heated, a reaction can occur with arsenic and chlorine, as well as phosphorus.
Calcium carbonate
The most important chemical quality is considered to be susceptibility to decomposition when exposed to water.
Hydrolysis or calcium carbide plus water
When calcium carbide interacts with water, a reaction occurs called hydrolysis. Hydrolysis of calcium carbide was once the main industrial method for producing acetylene, a flammable gas used in gas welding and gas cutting. You can learn about another production method from the article on the production of acetylene.
When calcium carbide (CaC2) reacts with water (H2O), a gas is obtained - acetylene (C2H2) and slaked lime (Ca(OH)2), which is a waste. The chemical activity of calcium carbide in relation to water is so great that it is decomposed even by the water of crystallization contained in salts.
The exothermic reaction (i.e. with the release of heat) of the interaction of calcium carbide with water proceeds rapidly according to the equation:
CaC2+2H2O=C2H2+Ca(OH)2 +30.4 kcal/mol
The thermal effect of the reaction consists of the heat released when calcium carbide and quicklime react with water. The interaction of lime with water proceeds according to the equation:
CaO+H2O = Ca(OH)2 +15.2 kcal/mol
Acetylene yield is the volume of acetylene in liters released during the decomposition of 1 kg of carbide, reduced to 20° and 760 mm Hg. Art.
To decompose 1 kg of chemically pure calcium carbide, 0.562 kg of water is theoretically required, which produces 0.406 kg of acetylene (285 l) and 1.156 kg of slaked lime.
The significant thermal effect of the reaction of calcium carbide and the danger of overheating of acetylene force the process to be carried out with a large excess of water for cooling. This makes the process safer. The temperature of the acetylene leaving the generator exceeds the ambient temperature by only 10-15°C.
Amount of water required to react with calcium carbide
The minimum amount of water required to cool the reaction of 1 kg of calcium carbide can be calculated as follows.
When 1 kg of 70% calcium carbide decomposes, 0.284 kg of acetylene and 1.127 kg of calcium oxide hydrate are formed, i.e. slaked lime (assuming the calcium oxide content in calcium carbide to be 24%).
We assume that the initial water temperature is 15° C, and the temperature in the generator during operation is 60° C. The heat balance equation for 1 kg of calcium carbide is expressed as follows:
q=q1+q2+q3+q4+q5
where q is the amount of heat released during the decomposition of 1 kg of 70% calcium carbide, equal to 397 kcal/kg q1 is the amount of heat spent on heating the resulting slaked lime from 15 to 60°C: q1= 1.127? (60-15) -0.23= 11.7 kcal 0.23 - average heat capacity of calcium oxide hydrate in kcal/kg
q2 - the amount of heat spent on heating the resulting acetylene from 15 to 60 ° C: q2 = 0.284? (60-15)-0.336 = 4.3 kcal 0.336 - average heat capacity of 1 kg of acetylene in kcal in the specified temperature range
q3 - heat spent on evaporation of water in the amount of 0.034 kg (at 60 ° C the content of water vapor saturating acetylene obtained from 1 kg of calcium carbide is equal to 34 g) latent heat of evaporation of water - 539 kcal / kg q3 = 0.034?539+ 0.034?1?(60-15) -19.9 kcal
q4 - heat loss to the environment and to heating the walls of the generator, it is approximately 7% of the total amount of heat released: q4 = 397? 7/100 = 27.8 kcal
q5 - amount of heat spent on heating water to a temperature of 60° C: q5=q?(q1+q2+q3+q4)=397?(11.7+4.3+19.9+27.8) = 336 .3 kcal
The required minimum safe volume of water is:
V=q5/(60-15)?1=336.3/45?7.5 l
Since 1 m3 of acetylene at an absolute pressure of 1 kgf/mm2 and 20°C weighs 1.09 kg, therefore, from 1 kg of chemically pure calcium carbide it is theoretically possible to obtain 0.406/1.09 = 0.3725 m3, or 372.5 l acetylene.
As mentioned above, technical calcium carbide usually contains no more than 70-80% CaC2. Therefore, from 1 kg of technical calcium carbide you can get from 230 to 280 liters of acetylene.
If we take into account the losses of acetylene due to dissolution in water and purging of the acetylene generator, then to obtain 1 m3 (1000 dm3) of acetylene it is practically necessary to consume 4.3-4.5 kg of calcium carbide. More accurate data on the actual yield of acetylene depending on the amount of impurities (grade) and the size of the “pieces” (granulation) are indicated in GOST 1460.
Parameters affecting the rate of reaction with water
The smaller the pieces, the faster the reaction of calcium carbide with water occurs.
Calcium carbide with a size of 50×80 mm decomposes completely within 13 minutes, and with a size of 8×15 mm - within 6.5 minutes.
When the size of the pieces is less than 2 mm, calcium carbide is considered waste and is called carbide dust. Carbide dust decomposes almost instantly. When interacting with water, the reaction of carbide dust occurs on the surface of the water and the heat generated cannot be quickly removed. This leads to an increase in temperature in the reaction zone and overheating of carbide particles and released acetylene. In this case, the presence of air is especially dangerous, since the ignition temperature of the acetylene-air mixture is quickly reached. Therefore, carbide dust cannot be used in conventional acetylene generators designed to operate on lump calcium carbide, as this may cause an explosion of acetylene in the generator. Specially designed generators are used to decompose carbide dust.
The higher the water temperature, the faster the calcium carbide reaction occurs. If the water is heavily contaminated with slaked lime formed during the reaction of calcium carbide, the reaction will slow down.
When immobile calcium carbide decomposes in an insufficient amount of water, pieces of it may become covered with a crust of slaked lime and become very overheated, and the following reaction may occur:
CaC2+Ca(OH)2 = C2H2+2CaO
In this case, the reaction of calcium carbide occurs due to the removal of moisture contained in slaked lime. As a result, the density of the crust increases, which leads to even greater overheating. Therefore, continuous removal of lime from the reaction zone is of great importance, since overheating can lead to an explosion of the acetylene-air mixture or cause explosive decomposition of acetylene.
If equal amounts of calcium carbide are decomposed by various gradually decreasing amounts of water, then the temperature of the resulting acetylene-water vapor mixture will correspondingly increase. At a temperature of about 90°C, almost all the heat (with the exception of the heat spent on heating acetylene and carbide sludge) is spent on the formation of water vapor. These reaction conditions correspond to the process by which dry calcium oxide hydrate is obtained, since all the water introduced into the reaction is spent on the decomposition of the carbide and the formation of water vapor.
When calcium carbide is immersed in water, the decomposition process also proceeds very unevenly: at first the reaction is very active with the rapid release of acetylene, and then the reaction rate decreases. This is explained by the reduction in the surface of the pieces and the fact that they are covered with a crust of lime, which prevents the free access of water.
When water is mixed with the calcium carbide in it, the reaction occurs faster and more uniformly.
The reaction rate of calcium carbide in water depends on the purity of the calcium carbide and the contact surface of the calcium carbide pieces with water.
The reaction rate of calcium carbide in water is a very important element that characterizes the quality of calcium carbide. For practical purposes, the concept of decomposition duration is used.
The duration of decomposition is considered to be the time during which 98% of the total amount of acetylene is released, which can be separated from calcium carbide, since the remainder decomposes very slowly and does not characterize the decomposition process in relation to the operating conditions of acetylene generators.
The table below shows experimental data on the duration of decomposition of calcium carbide depending on the size of its pieces.
| Piece sizes, mm | Dust | 2/4 | 5/8 | 8/15 | 15/25 | 25/50 | 50/80 |
| Decomposition time, min. | Few seconds | 1,17 | 1,65 | 1,82 | 4,23 | 13,5 | 16,6 |
It should be noted that these tables characterize only those calcium carbide samples with which experiments were carried out. In practice, significant deviations may occur, mainly in the direction of decreasing the reaction rate.
The rate of decomposition largely depends on the yield of acetylene from calcium carbide. The lower the yield, the slower the reaction rate.
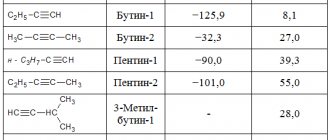
The chart below shows the changes in the decomposition rate of two grades of calcium carbide with the same lump sizes (25/50).
When decomposing 1 kg of calcium carbide with an acetylene yield of 263 l/kg, 220 l of acetylene are released in the first 3 minutes, and accordingly, with a yield of 226 l/kg, only 150 l.
Receipt
As previously noted, calcium carbide is actively used in the production of a wide variety of materials. That is why the process of obtaining calcium carbide has been constantly improved. The features of the technologies used include the following points:
- Quicklime is used as a raw material. In most cases, the substance is obtained from lime, but it is difficult to carry out such a procedure at home.
- Lime is mixed with crushed coke to obtain a homogeneous mass.
- In industry, calcium carbide is produced according to a scheme that involves heating the substance to a high temperature. Electronic ovens are used for this. The recommended melting point is 1900 ⁰C.
- After heating a substance to such a high temperature, it turns into a liquid state. Special forms are prepared for the work.
When considering how to obtain calcium carbide from carbon, we note that according to established standards, the composition must contain at least 80% of the main substance. The share of impurities should be no more than 25%, which also includes carbon. The production of calcium oxide also releases thermal energy, which is worth considering.
Production method
Carbide is produced in electric furnaces by fusing (calcining) a mixture of coke and calcium oxide (quicklime) at temperatures ranging from 1900°C to 2300°C. The sharp and unpleasant garlic smell of carbide and the produced acetylene is caused by the content of impurities of calcium phosphides and sulfates in the carbide mixture.
The process is carried out in several stages:
- Limestone is fired.
- A powder mixture is created from processed lime and coke - a mixture.
- The resulting mixture is calcined in an electric arc furnace until it melts.
- The resulting carbide bars are crushed to obtain the desired fraction.
The average density of the carbide substance is 2.2 g/cm3. Depending on the impurity content, the color of carbide can be dark brown or dark gray.
The final product consists of 75-78% CaC2, the rest being lime and impurities. Carbide granules come in different sizes: 2x8; 8x15; 15×25; 25x80 mm. Larger granules produce more acetylene but increase reaction time. If granules of 8x15 and 15x25 mm decompose in 5-6 minutes, then granules of 25x80 mm require more than 10 minutes to decompose.
Transportation and storage
Calcium carbide powder decomposes almost instantly when exposed to moisture. This produces acetylene, which in high concentrations is flammable and explosive. That is why it is necessary to pay quite a lot of attention to the storage of calcium carbide, for which cans and special drums are often used. Other storage features include the following:
- The released acetylene is lighter than air, so it accumulates at the top. It is worth considering that it has narcotic effects and can spontaneously ignite.
- When producing large volumes of a substance, special attention is paid to safety precautions. Special packaging is used for packaging.
- To open the package, use tools that do not cause sparks.
- If the substance gets on the skin or mucous membrane, it must be removed immediately. In this case, the affected surface is treated with a special cream or other protective and healing substance.
- According to established rules, transportation can only be carried out using a covered vehicle. However, delivery by air is prohibited.
Transport container
Regulations also prohibit storing calcium carbide with other chemicals and heat sources. This is because the resulting gases can react chemically with other chemicals and ignite.
Applications of calcium carbide
As previously noted, calcium carbide is found in a wide variety of industries and is often supplied for industrial synthesis. The properties of calcium carbide and the reaction that occurs when it is combined with various substances determine the use of the substance in the following cases:
- Many synthetic components that make up modern materials are produced on the basis of the component in question.
- Used to produce calcium cyanamide. A similar component is used to produce various chemical fertilizers. That is why raw materials are used to regulate the growth rate of plants.
- Calcium cyanamide is also obtained by combining the substance with nitrogen.
- In some cases, the reduction of alkali group metals is carried out.
- You can use the connection in question in the gas welding process.
When considering calcium carbide and its area of application, it is worth considering that such a substance is most often used to produce acetylene. A similar synthesis of calcium carbide was developed by a German scientist. Among the features of this method of application, we note the following points:
- Acetylene from carbide is obtained by exposing the raw materials used to water.
- As a result of the chemical reaction, the required gas is formed, and slaked lime precipitates.
- It is worth considering that when mixing components, a large amount of heat is generated. Therefore, work must be carried out taking into account safety precautions.
- Depending on the type of raw material processing technology used, about 290 liters of gas come out from 1 kilogram.
- The speed of the procedure depends on the purity of the raw materials used, temperature and amount of water.
Preparation of acetylene from calcium carbide
As practice shows, when using pure carbide, about 20 liters of water per 1 kilogram of raw material are allocated for the chemical reaction. A similar amount of water is required to lower the reaction temperature, thereby ensuring optimal operating conditions.
Acetylene production
One of the most important areas of application of calcium carbide is its use in the production of acetylene. The credit for the discovery of this method also belongs to the German chemist Friedrich Wöhler. This industrial process is based on the decomposition reaction of carbide under the influence of water. CaC2 + 2 H2O → C2H2 + Ca(OH)2↓. The output produces acetylene gas and slaked lime, which precipitates. The process is accompanied by the release of a large amount of heat. The volume of gas released depends on how pure the calcium carbide is used for the reaction. The resulting acetylene can have a different volume - 1 kg of the starting substance can produce from 235 to 290 liters of gas. As for the speed of the reaction, it depends both on the small percentage of impurities in calcium carbide and on the temperature of the water, as well as its purity. If we consider the theoretical reaction for the production of acetylene from carbide, then 560 ml of water is sufficient for 1 kg of carbide. However, in practice, the volume of water for the reaction increases. For 1 kg of calcium carbide under industrial synthesis conditions, 5 to 20 liters of water are required. This amount is necessary to ensure that the acetylene cools better and also to ensure optimal safety during operation. Below is the picture of German chemist Friedrich Wöhler.
Safety precautions
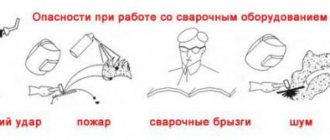
When carrying out various chemical reactions to produce materials, safety precautions must be observed. As previously noted, the released substances can be explosive. Safety precautions when interacting with various chemicals are as follows:
- An airtight place is required for storage and handling. It is not recommended to carry out work in a regular garage.
- Fire should not be allowed to reach the raw material itself, as well as the gases generated.
- Even small particles can damage the skin. That is why work must be carried out in a respirator and protective clothing.
- Acetylene generators are placed exclusively in well-insulated rooms.
- If the raw materials were used during welding work, then the resulting slag should be disposed of in special places.
- When moving metal and other containers, they must be securely fastened; collisions and falls are not allowed. This may result in sparks that could cause the substance to explode.
Calcium carbide combustion
The above information determines that work with the raw materials in question is not recommended to be carried out in a garage or home workshop. Failure to comply with technology, lack of required equipment and many other reasons can lead to sparks and ignition of substances.
Calcium carbide reaction with water
The raw material in question is most often used to combine with water, resulting in acetylene. The interaction of calcium carbide with water causes the appearance of gas with an unpleasant odor and a fairly large number of various impurities. Such a substance can only be obtained in its pure form through multi-stage purification.
The reaction of calcium carbide with water can be carried out experimentally. The features of such a procedure include the following points:
- A 1.5 liter bottle is used as a container.
- After filling it with water, a few pieces of crystalline material are added.
- The reaction leads to the appearance of excess pressure.
- Once the calcium carbide no longer reacts, burning paper is placed on the bottle. As a result of the interaction between calcium carbide and water, a gas is formed that explodes. During the experiment under consideration, a fiery cloud is formed.
Such an experiment is quite dangerous and must be carried out in compliance with safety precautions.
In conclusion, we note that the component in question has recently been often used to conduct a wide variety of experiments. The compound has a large number of properties that must be taken into account. The release of heat and gases becomes the reason why experiments are recommended only in industry.
Brief information
Carbides are chemical compounds that are composed of carbon (C) and another element. Usually the second element is metals - this can be iron (Fe), titanium (Ti), chromium (Cr), hafnium (Hf) and others. In carbide compounds, carbon has a higher electronegativity (compared to the second element). Therefore, carbides cannot be classified as oxides, halides and other chemical compounds. Metal carbides are mainly solid, refractory substances that are characterized by very high strength and almost do not react with chemically active substances.
Due to these unusual properties, carbides are often used as abrasives for processing various alloys (this can be cast iron, steel, aluminum-based compounds). They are used to make electrodes for welding, refractory rods, and elements of electrical systems. The first carbides based on potassium, iron and magnesium were discovered and obtained quite recently - in the 19th century. However, over time, new carbide compounds were obtained - based on chromium, titanium, hafnium, tungsten, and calcium.