The technology for producing most metal products is associated with plastic deformation and heating of the metal. In this case, the structure of the material and its structure-sensitive properties change significantly:
- strength;
- plastic;
- electrical conductivity;
Metals and alloys, with rare exceptions, are used in a polycrystalline state. A microscope shows that polycrystals consist of many grains - crystallites.
In polarized light, grains differ in their color due to different crystallographic orientation relative to the polished section plane.
Depending on the processing, grain sizes can vary greatly in size. From a few microns to several centimeters. A multi-grain structure may also form in the product.
The grains are separated by boundaries, the boundaries of which depend on the misorientation angle of neighboring grains. If these angles are greater than 10-15 degrees, the boundaries are called high-angle.
The grain size affects the properties of metals and alloys. Thus, metals with a fine-grained structure are characterized by increased strength and ductility. This is clearly visible in the tensile test. Coarse-grained metals are brittle and less strong.
The microstructure may differ in the nature of the crystallographic orientation of the grains. If most of the grains are closely oriented, then such a polycrystal is called textured.
Structures in which the crystallographic planes of a cube, pentahedron, or rhombic dodecahedron are located parallel to the rolling plane are very common.
A texture can contain a different number of texture components:
- two-component texture;
- one-component texture;
The nature of the texture affects the properties of the metal. The ductility of an untextured material is the same in all directions. That is, it is isotropic. In this case, the metal stretches uniformly during stamping.
The plasticity of textured metal is anisotropic. When stamping, the metal stretches unevenly.
The microstructure can be controlled using different methods of deformation and subsequent heating.
Process description
Essentially, everything is done in order to relieve internal tension between elements and increase density by creating the correct geometry of the joints.
Iron material is often cold processed in production. Rolled sheets and various types of wire are produced using this method. However, at a certain stage, the strength of the products decreases, as displacements accumulate and the bonds at the points of contact are significantly weakened.
Next, it is necessary to use recrystallization annealing to bring the metal to its primary state and, when applying a certain temperature (it is different for each alloy), to achieve changes (reducing fluidity and tensile strength and increasing ductility). The manipulation leads to the formation of new grains in the crystal lattice, which, with prolonged annealing with increasing heat treatment t, some newly formed, begin to grow at the expense of neighboring crystallites. Their size depends on the duration and thermal regime of the operation, that is, the longer the time spent. For iron alloys, heating is selected at the rate of 40 percent of the melting point. In this state, atoms acquire that degree of mobility and such a value of their own energy that it becomes possible to move and take the most advantageous position in the location area.
In addition, you need to know that the process of onset of action is directly related to the amount of heat treatment through a coefficient that has a different parameter for various alloys:
- material with the addition of a small amount of impurities has 0.4;
- high-frequency steel – 0.1-0.2;
- carbide solution - from 0.5 to 0.8.
Information on exact values can be found in the technical literature on metallurgy.
Let's talk about the speed of the procedure. The final result of the transformation depends on this parameter. Since the operation is not fast, it is possible to stop it by cooling. This results in the cell sizes of the required parameters.
An important point in production is the regulation of the speed regime, at which the stage of collecting grains of a given size and shape with their specific orientation is possible. To achieve the desired result, various additives (sulfur, manganese and other substances) are often used. The use of catalysts makes it possible to obtain a material with the necessary mechanical and physical properties.
Recrystallization
* primary, when new undistorted crystallites are formed in the deformed material, which grow, absorbing grains distorted by deformation;
* collective - undistorted grains grow at the expense of each other, as a result of which the average grain size increases;
secondary recrystallization, which differs from collective recrystallization in that only a few of the undistorted grains have the ability to grow. During secondary recrystallization, the structure is characterized by different grain sizes (heterogeneity).
The term “collective recrystallization” also corresponds to the term normal (that is, ordinary) grain growth.
Recrystallization eliminates structural defects (primarily reduces the dislocation density by several orders of magnitude), changes the size of grains and can change their crystallographic orientation (texture). Recrystallization transforms a substance into a state with greater thermodynamic stability: during primary recrystallization - by reducing distortions introduced by deformation; during collective and secondary recrystallization - due to a decrease in the total surface of grain boundaries. Recrystallization changes all structure-sensitive properties of the deformed material and often restores the original structure, texture and properties (before deformation). Sometimes the structure and texture after recrystallization differ from the original ones, and the properties also differ accordingly.
Recrystallization is widely used to control grain shape, size, texture and properties.
In steels, recrystallization is combined with spheroidization of cementite. The result is round cementite particles measuring 0.5-2 microns. This structure is called the tempering sorbitol structure. The heat treatment leading to it is an improvement.
In metallurgy, a simple rule is often used to determine the recrystallization temperature of an alloy: 0.4 of the melting temperature is taken as its value. Usually this approximation turns out to be quite sufficient.
The formation of recrystallization nuclei and the associated sharp change in properties characterize primary recrystallization, or processing recrystallization.
Increasing the holding time at the recrystallization temperature or further increasing the heating temperature leads to an increase in recrystallization centers. This process is greatly influenced by the processes of self-diffusion of atoms, and therefore the processes of grain growth during recrystallization have much in common with grain growth during a polymorphic transformation.
Recrystallization stages
In metallurgy, three phases of this method are used:
- Primary processing promotes the formation of new undistorted grains and the formation of areas that will be freed from dislocations or more perfect than the surrounding matrix (seeds grow due to its distortions). The restoration of the structure and qualities of undeformed raw materials occurs most radically.
- The collecting stage is characterized by the growth of crystallites due to currents occurring within the grains themselves. Due to a decrease in their length, a decrease in the energy level of the boundaries of elements is observed.
- Secondary is characterized by the creation of a multi-grained structure using various chemical compounds, as an example, manganese sulfide.
Return and recrystallization
The nonequilibrium structure created by cold deformation is stable at 25°C for most metals. The transition of the metal to a more stable state occurs when heated. As the temperature increases, the movement of point defects accelerates and conditions are created for the redistribution of dislocations and a reduction in their number.
The processes that occur during heating are divided into recovery and recrystallization. In turn, when returning, a distinction is made between rest and polygonization.
Return
call all changes in the fine structure and properties that are not accompanied by a change in the microstructure of the deformed metal, i.e. the size and shape of the grains do not change when returned.
Recrystallization
— this is the process of nucleation and growth of new grains with fewer structural defects; As a result of recrystallization, new, most often equiaxed grains are formed.
Rest
cold-deformed metal is called the recovery stage, at which the number of point defects, mainly vacancies, decreases;
in a number of metals (Al, Fe), rest also includes the creep of dislocations, which is accompanied by the interaction of dislocations of different signs and leads to a noticeable decrease in their density. The redistribution of dislocations is accompanied by a decrease in residual stresses. Rest reduces electrical resistivity and increases the density of the metal. Hardness and strength decrease by a maximum of 10 - 15 %
of the original values and ductility increases accordingly by the same amount. After resting, resistance to corrosion cracking increases.
Polygonization
is the process of formation of subgrains separated by low-angle boundaries. Each subgrain is a polyhedron, practically free of dislocations. Polygonization is the result of several elementary processes of dislocation movement: sliding and climbing of edge dislocations, transverse sliding of screw dislocations. During polygonization, the dislocation density decreases somewhat due to the interaction and annihilation of dislocations of opposite signs. To begin polygonization in cold-worked metals of technical purity, heating to 0.3...0.35 T (melt) is necessary, and in cold-worked alloys - to higher temperatures.
There are pre-recrystallization and stabilizing polygonization. Pre-recrystallization polygonization develops in cold-worked metals with a cellular dislocation structure. When heated, dislocation walls become denser and the cells turn into subgrains.
The compacted cell walls retain significant curvature and are so mobile that individual subgrains can enlarge and become centers of primary recrystallization. Pre-recrystallization polygonization is the initial stage of primary recrystallization. The structure of subgrains and their boundaries depends little on temperature. As the heating temperature of the cold-worked metal increases, the rate of polytonization increases: the polygonization structures formed at different annealing temperatures are practically the same.
Stabilizing polygonization is the formation of subgrains separated by flat dislocation walls (Fig. 5.12). The walls are inactive and very stable; with further heating they are preserved almost to the melting temperatures of metals. After the formation of the subgrain structure, recrystallization does not occur. Stabilizing polygonization develops only under certain conditions: the absence of a cellular dislocation structure, an excess of edge dislocations of the same sign, etc. Such conditions are met in single crystals and coarse-grained polycrystals after small plastic deformations. In such materials, the results of dislocation redistribution depend significantly on the annealing temperature. At relatively high heating temperatures (above 0.35 T(melt)), instead of polygonization, primary recrystallization develops. If the stabilizing polygonization is successfully completed after annealing at (0.3 - 0.35) T (melt)), then upon further heating, even at a higher temperature, recrystallization does not develop.
Limitation of dislocation mobility makes polygonization difficult. The fixation of dislocations by atoms of alloying elements and impurities, the formation of stacking faults, a decrease in the concentration of vacancies (the creep of dislocations is made more difficult) - all this complicates polygonization. It is more often observed in metals with high energy stacking faults (Al, Mo).
The practical significance of polygonization is manifested in the following.
1. The creation of a subgrain structure strengthens the metal by analogy with the formation of a fine-grained structure with high-angle boundaries. The effect of hardening during polygonization manifests itself on a smaller scale, since subgrain boundaries are able to more easily pass dislocations compared to high-angle boundaries.
2. The formation of a subgrain structure, while maintaining the main share of hardening of the cold-worked metal, reduces residual stresses. This increases resistance to corrosion cracking. In particular, for cold-worked brasses containing (20 - 35)% Zn, annealing is prescribed at ~ 300 °C to prevent cracking.
3. Subgrain boundaries are an obstacle to the movement of dislocations. This is used to increase the heat resistance of parts.
4. Subgrain structure formed during dynamic polygonization, i.e. during the deformation process, it provides an optimal combination of ductility and high strength during thermomechanical processing of steels.
Depending on the heating and holding temperature, three stages of recrystallization are distinguished: primary, collective and secondary.
Primary recrystallization
begins with the formation of nuclei of new grains and ends with the complete replacement of the work-hardened metal with a new polycrystalline structure.
At the stage of primary recrystallization, the nucleation and growth of new grains occur simultaneously. Grains grow by moving high-angle boundaries through the cold-worked metal. In such a grain the density of dislocations and other defects is minimal, while in cold-worked metal it is maximum.
Primary recrystallization ends when new grains completely replace the entire volume of deformed metal (see Fig. 5.13, b).
Primary recrystallization will completely remove the hardening created during plastic deformation, the metal acquires an equilibrium structure with a minimum number of defects in the crystalline structure. The properties of the metal after recrystallization are close to the properties of the annealed metal (Fig. 5.14).
Of particular importance is the growth of large grains when heating a deformed metal, when its deformation is close to critical. At critical deformation, a cellular dislocation structure is not yet formed, capable of creating recrystallization nuclei, which would contribute to the formation of a fine-grained structure. The heterogeneity of grain deformation and differences in the energy of elastic distortions are the driving force for the coarsening of grains due to less stable small grains.
Collective recrystallization
represents a spontaneous process of coarsening of grains formed at the stage of primary recrystallization. The larger the grains, the smaller the total surface area of the grain boundaries and the smaller the reserve of excess surface energy (compared to the volume of the grains).
Grain growth occurs as a result of the transfer of atoms from one grain to an adjacent one across the interface; At the same time, some grains gradually decrease in size and then completely disappear, others become larger, absorbing neighboring grains (Fig. 5.13, d). With increasing temperature, grain growth accelerates.
Collective recrystallization is inhibited when the grains become polyhedra with flat faces, and the angles between adjacent faces are 120° (Fig. 5.13e ).
Secondary recrystallization
represents a stage of uneven growth of some grains compared to others. As a result, a conglomerate of giant grains is formed adjacent to dwarf grains. The mechanical properties of such a heterogeneous structure are worse than those of a homogeneous structure of recrystallized metal. Secondary recrystallization corresponds to high heating temperatures of the cold-worked metal.
The recrystallization process described is typical of heating rates in conventional thermal furnaces, and exposures of the order of several hours are required to complete one or another stage of recrystallization.
Primary recrystallization is accelerated at high (~1000 °C/s) heating rates, where it develops at high temperatures and ends with the formation of a fine-grained structure in seconds instead of hours. To implement high-speed recrystallization, induction heating or direct passage of electric current through the cold-worked metal is used.
The plasticity and toughness of metals and alloys depend on the grain size. As the grain size decreases, the viscosity improves. The size of the grains formed as a result of recrystallization depends mainly on the degree of plastic deformation (Fig. 5.15, a), as well as on the temperature at which recrystallization occurred. Increasing the exposure time during heating promotes grain growth, but the effect is much less than with increasing heating temperature.
The dependence of grain size on the degree of deformation and temperature is demonstrated using recrystallization diagrams (Fig. 5.15, b).
For general purpose structural materials, anisotropy of properties is undesirable. Recrystallized alloys, as a rule, are homogeneous in properties and do not exhibit anisotropy. However, under certain conditions, a preferred crystallographic orientation of grains appears in the recrystallized metal, which is called the recrystallization texture.
Its type depends on the chemical composition of the alloy, the nature of deformation, the nature and amount of impurities, and technological factors.
Often it is a copy of the deformation texture of cold-worked metal. The formation of a recrystallization texture is of practical importance for alloys with special physical properties, when it is necessary to improve the properties in a certain direction of the product. For example, in transformer steel sheets, the formation of texture makes it possible to reduce losses due to magnetization reversal in certain directions of the sheet.
Recrystallization of multiphase alloys is a more complex process in which the nucleation and growth of new recrystallized grains is affected by differences in the properties of each phase, the nature of the structure, and the volumetric ratios between the phases. Of particular importance are the particle size of the second phase and the average distance between particles. The closer the particles of the second phase are located to each other, the more difficult it is for the boundary of a new grain to move and the more strongly recrystallization is inhibited. This is manifested in an increase in the recrystallization temperature and an increase in the time to complete the primary recrystallization of a multiphase alloy compared to a single-phase alloy. The proximity of particles of the second phase is ensured when their content in the alloy is sufficiently high. When particles are few and far between, their role in recrystallization is negligible. Small particles (0.1 µm or less) inhibit recrystallization (Fig. 5.16). Larger particles (over 0.1 - 0.5 µm) slow down recrystallization when they are located close to one another, and accelerate it when the distance between them increases (see Fig. 5.16). In the latter case, the influence of the interphase boundary is felt, at which new grains predominantly nucleate.
The inhibitory effect of dispersed particles of the second phase on recrystallization is successfully used in industrial alloys to increase operating temperatures.
During hot deformation of materials with ultrafine grains (0.5 - 10 microns), the superplastic state of the metal appears.
At low strain rates (10e-5 – 10e-4 s(-1)), the metal flows uniformly without hardening: relative elongations reach 10e2 – 10e3
%.
Enormous deformations in the superplastic state consist of grain-boundary sliding, supplemented by directed (under the influence of stress) diffusion transfer of atoms and ordinary sliding within grains. In order to realize the superplastic state, it is necessary to preserve ultrafine grains throughout the entire period of deformation (on the order of tens of minutes) at a temperature above 0.5 T (melt). Industrial superplastic alloys have a two-phase structure (the best combination of the volumes of both phases is 1:1, since this maximizes the surface area of the interphase boundaries) and therefore retains the original fine grain throughout the entire production period of the products. Such alloys include various eutectic and eutectoid mixtures, two-phase titanium alloys, etc.
The superplastic state is used in practice for the production of products of very complex shapes using pneumatic sheet molding or volumetric pressing. Despite the slowness of the molding process itself and the relatively high operating temperatures, the process is profitable, and in some cases it is the only way to obtain products when the metal needs to be deformed by 200 - 300 without destruction. %
and higher.
Processes based on the use of a fine-grained structure are widely used in industry. Superplasticity is observed during hot deformation of alloys in close proximity to the temperatures of polymorphic transformation or melting. In these cases, the microstructure is preserved, but the crystal lattice of the alloy base turns out to be unstable: for example, the elastic modulus decreases by 2–3 times. At low deformation rates, the metal is capable of deforming by tens of percent without destruction.
The process of plastic deformation (recrystallization) of a metal
This method achieves a change in the original geometric shape and size after removing the mechanical load on the body, and is accompanied by a change in the distance between the atoms in the crystal lattice within its parameter. Simultaneously with this operation, internal stress of the material occurs, which ultimately leads to a transformation of physical and chemical properties. The degree of deformation depends on the ductility of the alloy, which is assessed during production by the relative expansion or contraction when testing samples in tension. The characteristics also include impact strength, which shows the work of destruction during bending of the model.
Additional Information! Plasticity depends on the difference between tensile strength and yield. Almost identical values (when heated to high temperatures) contribute to the destruction of brittle materials virtually without plastic deformation. These include cast iron, glass, ceramics, porcelain, some types of plastic, rocks and others.
35. Return, primary and collective recrystallization. Recrystallization annealingAbout 10–15% of the total energy expended on plastic deformation is absorbed by the metal and accumulates in it in the form of increased potential energy of displaced atoms and stresses. The deformed metal is in a nonequilibrium, unstable state. The transition to a more equilibrium state is associated with a decrease in distortions in the crystal lattice and the release of stress, which is determined by the possibility of movement of atoms. At low temperatures, the mobility of the atom is low, and in the hardened state it can persist indefinitely.
With increasing temperature, the diffusion of atoms increases and processes begin to develop in the metal, leading it to a more equilibrium state. This is a return phenomenon.
The first stage of return is rest, observed at low heat. During rest, the number of vacancies decreases, the dislocation density decreases, and stress is partially relieved.
The second stage of return is polygonization, dividing grains into parts - polygons (subgrains).
Polygonization occurs as a result of sliding and crawling of dislocations, as a result of which dislocations of the same sign form “walls” that separate grains into polygons. In a polygonized state, a crystal has less energy compared to a deformed one, and the formation of polygons is an energetically favorable process. The temperature at which polygonization begins is not constant. The speed of polygonization depends on the nature of the metal, the degree of previous deformation, and the content of impurities. Upon return, no noticeable changes in the microstructure are observed; the metal retains its fibrous structure. At the same time, hardness and strength decrease somewhat, and ductility increases.
Recrystallization. When heated to sufficiently high temperatures, the mobility of atoms increases and the process of recrystallization occurs.
Recrystallization is the process of formation and growth of new grains when the cold-worked metal is heated to a certain temperature. This process occurs in two stages. A distinction is made between primary recrystallization (processing) and collective recrystallization.
Primary recrystallization (processing) consists of the formation of nuclei and the growth of new equilibrium grains with an undistorted crystal lattice. It is most likely that new grains arise at the boundaries of blocks and grains, slip packets within grains, where the metal lattice was most severely distorted during plastic deformation. The number of new grains gradually increases and, ultimately, no old deformed grains remain in the structure.
A deformed metal in an unstable state tends to transition to a stable state with the least amount of free energy. This state corresponds to the process of formation of new grains with an undistorted crystal lattice. In places where the lattice is most distorted and, therefore, least stable, when heated, atoms move, the lattice is restored, and nuclei of new equilibrium grains appear. The nuclei of new grains can also be volumes (blocks) with the least distorted lattice, into which atoms move from neighboring volumes with a distorted lattice.
Collective recrystallization - the second stage of the recrystallization process consists of the growth of new grains formed. The driving force for collective recrystallization is the surface energy of the grains. The growth of grains is explained by the fact that if there are a large number of small grains, their total surface area is very large, so the metal has a large supply of surface energy. As grains become larger, the total length of their boundaries becomes smaller, which corresponds to the transition of the metal to a more equilibrium state.
With the onset of recrystallization, a significant change in the properties of the metal occurs, which is opposite to the change in properties during hardening. The strength of the metal decreases. Plasticity, viscosity, thermal conductivity and other properties increase, which decrease during hardening. The properties of the metal are greatly influenced by the size of the grains obtained during recrystallization. The grain size increases with increasing holding time. The largest grains are formed after slight preliminary deformation. This degree of deformation is called critical.
Recrystallization annealing. This type of annealing is carried out to eliminate the hardening of cold-deformed metal. The cold-worked metal is very hard and brittle; its crystal lattice is in a nonequilibrium state, possessing a large supply of excess free energy. In heavily cold-worked metal, due to the merging of dislocations in places where they accumulate, dangerous defects are observed - crack nuclei. In some cases, hardening has to be eliminated. This requires heating to stimulate diffusion processes. However, recrystallization annealing is more preferable due to its significantly lower temperature and much shorter duration with almost identical results.
Table of contents
Structural changes in metal when heated
The deformed sample is in a nonequilibrium state, so further processing is necessary using elevated temperatures. This method allows you to move to equilibrium by reducing distortions in the lattice, namely, removing stress for the free movement of atoms. As a result, when heated, new undistorted crystallites of the initial phase nucleate and grow. And this, in turn, leads to a transformation not only of the microstructure, but of their properties. After these manipulations the following occurs:
- A sharp decrease in strength with a simultaneous increase in ductility.
- Reducing resistance to electrical conductivity.
- Increased thermal conductivity.
These parameters are characteristic of iron in which small grains have formed. Coarse-grained materials have high magnetic characteristics and physical properties begin to resemble cast iron.
Description of primary recrystallization
Deformed metal or fresh rolled metal undergoes a natural process of forming cells with the most energetically favorable shapes. The physical impact displaces the layers, while the structure is subject to tension and, conversely, compression at other points. This imbalance tends to return to its natural normal state. At room temperatures and minimal heating, these events occur at a very low rate, since there is not enough vibrational motion of the atoms. Sharp acceleration occurs when internal energy increases. The optimal indicator depends on the weight of the primary element and the degree of connection with its neighbors, that is, on the chemical composition.
Plastic deformation and recrystallization
Plastic deformation
Changes in the shape of grains during deformation occur mainly through the movement of dislocations along slip planes. The atomic mechanism of this process is associated with the formation and sliding of dislocations. Shear lines are where gliding dislocations accumulate.
As strain increases, dislocations move in new slip systems. The dislocation density increases. The interaction of stress fields around a dislocation causes their complex entanglements. At the same time, the metal is strengthened and becomes less ductile.
Changes in the dislocation structure inside a grain can be observed using an electron microscope, with a magnification of tens of thousands of times. In this case, dislocations are visible as dark lines, and dislocation clusters as dark areas.
At the first stage of hardening, dislocations move mainly in one slip system.
In the second stage, sliding begins in several systems.
At the third stage, complex entanglements of dislocations are formed and a cellular structure appears. Cellular structure, the most important feature of the third stage of hardening.
In the volume of cells, the dislocation density is relatively low. Basically, they are concentrated in the walls of the cells. The wall thickness can vary from several hundred angstroms to several fractions of a micron.
Thin walls are characteristic of metals with high stacking fault energy. Blurred walls are characteristic of metals with low energy stacking faults.
In the middle of the grain, the cells are slightly misoriented relative to each other. Near the grain boundary, the misorientation is much stronger. If the sliding of dislocations is hindered, deformation occurs through twinning. In this case, the crystallite atoms naturally shift relative to the twinning plane.
What is secondary recrystallization
We must understand that the movement of atoms is a chaotic action, in which the overall tension between fragments tends to a minimum. Therefore, after the “production” of orphan particles, grain growth begins only in areas where this is possible. At the same time, there is a consolidation of some and stabilization of others. As a result, we see matter with cells of different sizes. As in the first point, ordering of space is observed with a decrease in the surface area of the crystals. This also leads to a change in physical properties, although less pronounced than in the previous case.
Sintering. Recrystallization. Part 11
The influence of soluble impurities is especially significant. By changing the forces and energy of interatomic interaction in the body, soluble impurities also change the activation energy
diffusion phenomena, and consequently, the recrystallization process. In this case, an impurity that increases the energy of interatomic interaction (which occurs in the vast majority of cases) naturally reduces the mobility of atoms and slows down diffusion and recrystallization [170].
Preliminary deformation of the body (increasing the degree of deformation), affecting the whitefish values, especially the second of these values, in most cases accelerates recrystallization.
In a polydisperse body, large grains, which have lower surface energy, grow especially intensively during primary recrystallization.
Both the rate of nucleation and the rate of crystal growth are very sensitive functions of temperature. At a certain degree of body deformation, the dependence of the growth rate on temperature can be approximately expressed by the formula
where is the activation energy for crystal growth.
The same is the nature of the dependence of the value of ha on temperature, and it is known that only at small deformations (<5%)
Finally, it has been established that at a constant temperature, the rate of nucleation of crystal centers r increases significantly as the process progresses, while the average growth rate c in most cases remains virtually unchanged with time [171–174].
Often they talk about the temperature of recrystallization or the beginning of recrystallization characteristic of a substance. By these names we should understand the temperature of a noticeable speed of the process under consideration (i.e., a sharp, abrupt increase in this speed), depending, as is clear from the above, on many factors, in particular on the degree of deformation of the heated material. As the latter increases, the recrystallization temperature decreases.
According to Bochvar, during recrystallization, as well as during melting, the amplitude of vibrations required to carry out the process is a certain fraction of the crystal lattice constant (naturally, smaller for recrystallization than for melting). Based on this, Bochvar [175, 176] came to the conclusion that, without taking into account complicating circumstances, the recrystallization temperature of different metals should be the same fraction of their melting temperature. Limpton came to the same conclusions [177]. This corresponds to the fact, noted by Tamman, that the mobility of the elements of the crystal lattice (causing, for example, the sintering of substances) increases sharply at a temperature that is a certain fraction of the absolute temperature of these substances (at the Tammann temperature). The magnitude of this relative temperature varies
Other parts:
Sintering. Recrystallization. Part 1
Sintering. Recrystallization. Part 2
Sintering. Recrystallization. Part 3
Sintering. Recrystallization. Part 4
Sintering. Recrystallization. Part 5
Sintering. Recrystallization. Part 6
Sintering. Recrystallization. Part 7
Sintering. Recrystallization. Part 8
Sintering. Recrystallization. Part 9
Sintering. Recrystallization. Part 10
Sintering. Recrystallization. Part 11
Sintering. Recrystallization. Part 12
Sintering. Recrystallization. Part 13
Content
Metallurgical effects of cold working
Changes in properties under physical influence at temperatures below a certain value have been used at all times to obtain the required product parameters. Due to deformation, internal stress increases, which entails an increase in strength and flexibility. In addition, viscosity is reduced. Excessive influence leads to cracks and peeling. The greatest effect is observed in the top layer of large workpieces.
Results of recrystallization annealing
During forging, the structures in the metal are partially destroyed, and zones with an amorphous state appear. As described earlier, prolonged temperature exposure allows, by obtaining a certain degree of freedom, to start the process of ordering the spatial position of individual elements. The displaced areas are filled with newly formed grains. In this case, the properties of the raw material before forging are gradually restored. A further increase in heating leads to the enlargement of formations.
Another reason for metal recrystallization in materials science by annealing
This technique is also widely used because by selecting time and temperature, you can smoothly change the parameters of the feedstock. In this case, the transformation occurs quite smoothly, which means that the achievement of the specified properties can be predicted. The simplest example would be the annealing of steel wire to a “knitting” state by simple short-term heating to a burgundy color.
Illustration of changes using tantalum alloy as an example
This material has very characteristic transformations during this kind of manipulation, since it is quite widely in demand in prosthetics, protecting especially important structural components from corrosion and the influence of aggressive environments. We chose it to visualize typical processes.
Mechanical impact forms clearly noticeable zones with a damaged structure. Due to the lack of stable crystalline bonds, these areas actively enter into chemical reactions with a wide range of reagents. The product itself acquires increased fragility and a heterogeneous internal structure with clearly defined areas of shear. During heat treatment, chaotic areas are filled with newly initiated formations until the damage is completely filled. This is what is called texture recrystallization. In this case, the alloy goes through a very slow path to its previous state. Stopping the action at a certain point in time allows you to fix the properties. Usually carried out until the smallest area of contact between the grains is achieved. This situation is characterized by a very low tendency to oxidation, since the interatomic bonds existing in the ordered structure of crystals do not allow interaction even with very strong oxidizing agents.
Corrosion and conductivity
Tantalum alloy is applied in a thin layer to the surface of the supporting structure.
With this action, zones with an amorphous or semi-amorphous state are formed, which are especially dangerous centers of oxidation. To eliminate defects, crystallization and recrystallization are carried out. The entire product is heated and held for a specified time. This operation allows you to obtain the correct structure in problem areas. The geometric accuracy of the position of the nuclei in the grains, as well as minimal thermal fluctuations, ensure the unhindered passage of electrons under the influence of the field. This allows us to sharply reduce the number of collisions, and hence the heating (resistance) of the material. The larger the formations, the higher the conductivity.
Methods for assessing change
For the experiment, a sample was taken, deformed in a rolling machine. The workpiece was reduced from five millimeters to 1.2 mm. This made it possible to obtain characteristic structural disturbances. After this, the second sample was held at a temperature of 1200 degrees Celsius for ten minutes and the second sample at 1350° for 240 minutes. Both petals are sanded, degreased and cleaned. Access zones are formed on them (a fixed area is left clean, everything else is isolated). After this, they were exposed to aggressive solutions (sulfuric acid, hydrofluoric acid and methyl alcohol as a solvent). Cathode and anodic reactions were carried out with current measurements.
Microstructures and results
Based on the data obtained during testing, the following conclusions can be drawn:
- The corrosive surface of the deformed sample increased noticeably due to the development of easily reacting zones that do not have a clearly defined crystalline structure.
- Fragments that had previously undergone the recrystallization procedure showed a linear response to influence and virtually uniform interaction over time.
- The second sample with an enlarged crystalline system turned out to be the most resistant to both anodic and cathodic effects. Moreover, after a given time period, the surface retained its uniformity without the formation of cavities and grooves.
Detailed calculations and subtleties of organizing this experiment can be found in scientific works on metal science. There, all actions to achieve the purity of experience are scrupulously shown and reports are presented in numbers about all the stages carried out.
For everyday use, it is most interesting to know what the recrystallization temperature of steel and pure metals is, since this allows you to radically change the properties of objects through simple manipulations. For example, change the spring parameters, harden or release the cutter. To reinforce the material, watch the video:
The influence of recrystallization temperature on the structure and properties of cold-deformed metals
⇐ PreviousPage 7 of 12Next ⇒
The nonequilibrium structure created by cold deformation is stable in most metals at a temperature of 25 ºС. The transition of the metal to a more stable state occurs when heated.
The processes occurring during heating are divided into two main stages: recovery and recrystallization.
The removal of lattice distortions as a result of numerous submicroprocesses (a decrease in the density of dislocations as a result of their mutual destruction - annihilation, a decrease in internal stresses, a decrease in the number of vacancies, etc.) during the heating of a deformed metal is called return or rest. Upon recovery, there is no noticeable change in the structure visible under a light microscope compared to the deformed state. Return occurs at relatively low temperatures (about ).
Recrystallization is the nucleation and growth of new grains with fewer structural defects; As a result of recrystallization, completely new, most often equiaxed grains are formed. The recrystallization temperature is a certain fraction of the melting temperature of the metal: and is the lowest heating temperature that allows the nucleation of new grains.
The recrystallization temperature can fluctuate and depends on:
on the degree of deformation (the lower the deformation, the higher the recrystallization temperature;
on the holding time during heating (the longer the holding time, the lower the recrystallization temperature);
on the purity of the alloy (the more impurities in the alloy, the higher the recrystallization temperature).
The nucleation of new grains during recrystallization occurs in areas with the highest dislocation density, usually at the boundaries of deformed grains. Over time, the formed centers of new grains increase in size due to the transition of atoms from a deformed environment to a more perfect lattice. This recrystallization step is called primary recrystallization or processing recrystallization. Primary recrystallization ends when new grains completely replace the entire volume of deformed metal (Fig. 5c).
Rice. 5. Scheme of changes in the microstructure of cold-worked metal during heating:
a) cold-worked metal; b) the beginning of primary recrystallization;
c) completion of primary recrystallization;
d), e) stages of collective recrystallization
Upon completion of primary recrystallization, the resulting grains grow with increasing holding time or temperature; this stage of recrystallization is called collective recrystallization. The higher the heating temperature, the larger the recrystallized grains will be.
Primary recrystallization completely removes the hardening created during plastic deformation; the metal acquires an equilibrium structure with a minimum number of defects in the crystalline structure. The properties of the metal after recrystallization are close to the properties of the annealed metal (Fig. 6).
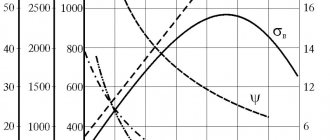
The plasticity and toughness of metals and alloys depend on the grain size. As the grain size decreases, the viscosity increases. The size of grains formed during recrystallization depends mainly on the degree of plastic deformation, on the temperature at which recrystallization occurs, and on the holding time (Fig. 7).
Increasing the holding time during heating promotes grain growth, but this effect is much less than when the heating temperature increases.
From Fig. 7c it is clear that at a certain degree of deformation, the so-called critical deformation (3÷5%), and recrystallization, anomalously large grains can be obtained. A critical degree of deformation should be avoided, since the resulting coarse-grained structure has reduced ductility and impact toughness.
At large degrees of deformation, many centers of new grains appear, and after recrystallization, a fine-grained polycrystal with good mechanical properties is formed.
Rice. 6. Schemes of changes in hardness (a) and plasticity (b)
cold-worked metal when heated: I – return,
II – primary recrystallization, III – grain growth
Rice. 7. Effect of temperature (a), heating duration (b) and degree
deformation (c) by the amount of recrystallized grain.
On, On' – incubation period of recrystallization;
– temperature of recrystallization annealing;
, – critical degree of deformation
Work order
1. Measure the thickness of the samples before and after deformation.
2. Perform cold plastic deformation on a machine with forces of 6, 8, 10 tons.
3. Measure the hardness of the samples after deformation using a Rockwell device on a B scale (HRB) and enter it into the table.
4. Calculate the degree of plastic deformation of the samples:
.
5. Calculate the tensile strength of deformed samples using the empirical relation.
6. Anneal the deformed samples at temperatures of 250, 400 ºС with holding time for 15 minutes.
7. Measure the hardness of the samples after recrystallization annealing.
8. Study the structure of the samples after deformation and recrystallization, sketch and determine the grain number (album, p. 19, scale 1).
Contents of the report
1. Purpose of the work.
2. Table with measurement results.
| P. No. | Load P, t | Sample thickness h, mm | , % | HRB | HB | Annealing temperature t, ºС | HRB | HB |
3. Graph of the degree of deformation ( ) versus load (P).
4. Graph of the dependence of tensile strength ( ) on the degree of plastic deformation ( ).
5. Graph of hardness (HRB) versus annealing temperature (t).
6. Structures of samples after deformation and annealing, indicating the grain number.
7. Conclusions from the constructed graphs.
6. Security questions
1. What is plastic and elastic deformation?
2. What is hardening of metals?
3. What is recrystallization, what stages does this process consist of?
4. How does the recrystallization temperature depend on the melting temperature of metals and alloys?
5. What is the critical degree of deformation?
6. Why does the grain size depend on the degree of deformation?
7. What changes occur in metals as a result of plastic deformation?
8. What factors influence the recrystallization temperature of metals?
9. What is meant by return or rest?
10. What factors and how do they affect the grain size after recrystallization?
Laboratory work No. 5
Hardening of carbon steels
Goal of the work
1. Master the methodology for selecting modes and technologies for hardening carbon steels of various compositions.
2. Study the diagram of the isothermal transformation of austenite.
3. Study the structure of hardened steel and explain its production using iron-carbon and isothermal transformation diagrams.
4. Study the effect of the amount of carbon and cooling rate on the hardness of hardened steel.
⇐ Previous7Next ⇒
Recommended pages: