Finding titanium in nature
Titanium occupies an honorable fourth place in terms of content in the earth's crust among metals important for humans, second only to iron, magnesium and aluminum. Its maximum amount is concentrated in the lower, basalt layer, and slightly less in the granite layer. Taking into account the high chemical activity, it is not possible to find titanium in its pure form. The most common are tetravalent oxides, which are concentrated in weathering crust ores and in marine clay.
Today there are up to 75 titanium minerals, and scientists periodically announce the discovery of new forms and compounds. For industrial processing, the following are of greatest importance:
- Ilmenite.
- Leucoxene (an alteration product of ilmenite).
- Rutile.
- Titanite (sphene).
- Perovskite.
- Anataz.
- Titanomagnetite.
- Brookite.
Titanium is a weak migrant; it can only be transported in the form of mechanical fragments of rock or during the movement of colloidal silty layers of reservoirs. The biosphere is characterized by the content of maximum quantities of this metal in seaweed; in animals it is found in wool and horny tissues; in the human body it is present in the thyroid gland, spleen, adrenal glands and placenta.
Is it possible to penetrate titanium? Titanium plate
[edit] Discovery history
In 1791, the English chemist and mineralogist William Gregor discovered a new element in the mineral menacanite and named it “menacanum.” German chemist Martin Klaproth rediscovered it in the mineral rutile in 1795 and gave it the fancy name titanium. Within 2 years, it turned out that Gregor and Klaproth had discovered the same element, which has since bear the majestic name - titanium. Titanium metal was first mined by Berzelius in 1825, but it was a very contaminated metal. Many scientists tried to obtain titanium in its pure form, and only in 1875 the Russian scientist D.K. Kirillov was for the first time able to obtain metallic titanium containing a few percent of the mixture. In 1910, the American chemist Hunter was able to produce a few grams of pure titanium, which contained a few tenths of a percent of mixtures that made it practically unsuitable for processing. And although titanium salts had already found use, it was only in 1925 that high-purity titanium, obtained by Dutch scientists Van Arkel and where Bure, demonstrated its unique properties.
Deposits of space material
The most common are ilmenite deposits, they amount to about 800 million tons. The reserves of rutile ores are much smaller, but if production continues to grow, all of them can provide humanity for another 100 years. In terms of titanium reserves, Russia is second only to China and has 20 explored deposits. Most of them are complex, where iron, phosphorus, vanadium and zirconium are also mined. Today, the Russian metallurgical industry is considered the world's largest titanium producer.
Extensive deposits are located in South Africa, Ukraine, Canada, the USA, Brazil, Australia, Sweden, Norway, Egypt, Kazakhstan, India and South Korea. They differ in the metal content in the ores and production volumes; geological surveys do not stop. Even on the Moon, reserves of titanium-containing ores have been discovered, some of them tens of times richer than large deposits on Earth. This allows us to hope for a reduction in market prices for the metal and an expansion in the scope of its use.
How to drill titanium and titanium alloys
Sources of titanium scrap
Based on regulatory documentation, titanium scrap is divided into:
- large without tin additives;
- waste from sheet material processing;
- unalloyed (pieces);
- shavings;
- lump scrap;
- metallurgy waste.
Bicycle frame made of titanium alloy
The category of industrial waste includes pure metal without foreign impurities and alloys in the form of lump scrap. These are elements of main pipelines, thickeners and vacuum filter housings, remains of titanium sheets, and parts of shut-off valves. The list must include technological elements of heat exchange equipment, various containers for transporting and storing chemically active substances, and equipment with rapid rotation units. The last category includes dryers, separators, centrifuges, compressors, and blades of various turbines.
The list of household products includes bicycle frames, car rims, watch cases and smartphone cases. Titanium is present in the structural elements of cars, and its alloys are used in parts of the chassis and internal combustion engines. Mufflers, connecting rods, valves and rocker arms can be made from heat-resistant grades of titanium compounds.
GOST includes shavings, trimmings of any workpieces, elements and pipes as processing waste. Recycled raw materials accepted under this category must not have dirt, oxidized surfaces, or paint or varnish residues.
Loose non-corrugated chips without any impurities are allowed for acceptance. Even inclusions in the form of small fragments of the cutting tool are not allowed. Any titanium scrap is twice as expensive as chips.
Reserves and production
Main ores: ilmenite (FeTiO3), rutile (TiO2), titanite (CaTiSiO5).
As of 2002, 90% of mined titanium was used to produce titanium dioxide TiO2. World production of titanium dioxide was 4.5 million tons per year. Confirmed reserves of titanium dioxide (excluding Russia) are about 800 million tons. As of 2006, according to the US Geological Survey, in terms of titanium dioxide and excluding Russia, reserves of ilmenite ores amount to 603-673 million tons, and rutile ores - 49, 7-52.7 million tons. Thus, at the current rate of production, the world's proven reserves of titanium (excluding Russia) will last for more than 150 years.
Russia has the second largest reserves of titanium in the world, after China. The mineral resource base of titanium in Russia consists of 20 deposits (of which 11 are primary and 9 alluvial), fairly evenly distributed throughout the country. The largest of the explored deposits (Yaregskoye) is located 25 km from the city of Ukhta (Komi Republic). The deposit's reserves are estimated at 2 billion tons of ore with an average titanium dioxide content of about 10%.
The world's largest titanium producer is Russian.
[edit] Distribution
The average titanium content in the earth's crust (clarke) is 0.45% (according to other data - 0.61% to a depth of 16 km). Only three other important metals—aluminum, iron, and magnesium—are more abundant in nature than titanium. The pegmatites of granites and alkaline rocks are richest in titanium.
By the beginning of the 21st century. About 100 titanium minerals are known. Titanium is included as an impurity in a number of minerals.
The main minerals of titanium ores: ilmenite (43.7-52.8% TiO 2); rutile, anatase and brookite (94.2-99.5); leucoxene (61.9-97.6); loparit (38.3-41); sphene (33.7-40.8); perovskite (38.7-57.8).
The amount of titanium in the earth's crust is several times greater than the reserves of copper, zinc, lead, gold, silver, platinum, chromium, tungsten, mercury, molybdenum, bismuth, antimony, nickel and tin combined. The titanium clarke in basic igneous rocks is 20.46 atomic %.
Characteristics and properties
The characteristics of titanium directly depend on the amount of impurities contained in its composition. Physical parameters:
- Specific strength - 450 MPa.
- The melting point of titanium is 1668 degrees.
- Boiling point - 3227 degrees.
- The tensile strength of the alloys is 2000 MPa.
- The elasticity of titanium is 110.25 GPa.
- Metal hardness - 103 HB.
- The yield strength is 380 MPa.
The structure and properties of this metal determine its low electrical conductivity. Under normal conditions, titanium has a high resistance to corrosion processes.
Physical properties of metal
Titanium is a silvery-white metal. It is refractory, slightly heavier than aluminum. However, with slightly more weight, titanium has three times the strength. Amenable to various processing methods. Resistant to moisture and acids. The main properties of titanium have been described above.
Metal density
The density of a substance varies depending on temperature and phase.
- At temperatures from 0 to the melting point it decreases from 4.51 to 4.26 g/cubic meter. cm, and during the phase transition it increases by 0.15%, and then decreases again.
- The density of liquid metal is 4.12 g/cubic. cm, and then decreases with increasing temperature.
Melting and boiling points
The phase transition divides all the properties of the metal into qualities that the α- and β-phases can exhibit. Thus, density up to 883 C refers to the qualities of the α-phase, and melting and boiling points refer to the parameters of the β-phase.
- The melting point of titanium (in degrees) is 1668+/-5 C;
- The boiling point reaches 3227 C.
It is one of the most heat-resistant metals known in metallurgy.
The following is a brief description of titanium with technical specifications. mechanical features.
The combustion of titanium is discussed in this video:
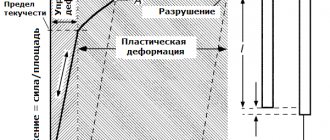
Mechanical Features
Titanium is approximately 2 times stronger than iron and 6 times stronger than aluminum, which makes it such a valuable structural material. The indicators relate to the properties of the α-phase.
- The tensile strength of the substance is 300–450 MPa. The indicator can be increased to 2000 MPa by adding some elements, as well as by resorting to special processing - hardening and aging.
It is interesting that titanium retains its high specific strength even at the lowest temperatures. Moreover, as the temperature decreases, the bending strength increases: at +20 C the indicator is 700 MPa, and at -196 – 1100 MPa.
- The elasticity of the metal is relatively low, which is a significant disadvantage of the substance. The modulus of elasticity under normal conditions is 110.25 GPa. In addition, titanium is characterized by anisotropy: elasticity in different directions reaches different values.
- The hardness of the substance on the HB scale is 103. Moreover, this indicator is average. Depending on the purity of the metal and the nature of the impurities, the hardness may be higher.
- The nominal yield strength is 250–380 MPa. The higher this indicator, the better the products made from the substance resist loads and the more they resist wear. The index of titanium exceeds that of aluminum by 18 times.
Compared to other metals that have the same lattice, the metal has very decent ductility and malleability.
Next, the specific heat capacity of titanium is considered.
Heat capacity
The metal has low thermal conductivity, therefore, in the relevant areas - the production of thermoelectrodes, for example, is not used.
- Its thermal conductivity is 16.76 l, W/(m × deg). This is 4 times less than iron and 12 times less than aluminum.
- But the coefficient of thermal expansion of titanium is negligible at normal temperatures and increases with increasing temperature.
- The heat capacity of the metal is 0.523 kJ/(kg K).
Electrical characteristics
As most often happens, low thermal conductivity also ensures low electrical conductivity.
- The electrical resistivity of the metal is very high - 42.1·10-6 ohm·cm under normal conditions. If we assume the conductivity of silver to be 100%, then the conductivity of titanium will be 3.8%.
- Titanium is a paramagnet, that is, it cannot be magnetized in a field, like iron, but it will not be pushed out of the field, like copper. This property decreases linearly with decreasing temperature, but after passing the minimum, it increases slightly. The specific magnetic susceptibility is 3.2 10-6 G-1. It is worth noting that susceptibility, like elasticity, forms anisotropy and varies depending on the direction.
At a temperature of 3.8 K, titanium becomes a superconductor.
Corrosion resistance
Under normal conditions, titanium has very high anti-corrosion properties. In air, it is covered with a layer of titanium oxide 5–15 microns thick, which ensures excellent chemical inertness. The metal does not corrode in air, sea air, sea water, wet chlorine, chlorine water and numerous other technological solutions and reagents, which makes the material irreplaceable in the chemical, paper-making, and oil industries.
When the temperature rises or the metal becomes very crushed, the picture changes dramatically. The metal reacts with almost all the gases that make up the atmosphere, and in the liquid state it also absorbs them.
The toxicity of titanium is discussed next.
Safety
Titanium is one of the most biologically inert metals. In medicine, it is used for the manufacture of prosthetics, as it is resistant to corrosion, lightweight and durable.
Titanium dioxide is not so safe, although it is used much more often - in the cosmetics and food industries, for example. According to some data - UCLA, research by pathology professor Robert Schiestle, titanium dioxide nanoparticles affect the genetic apparatus and can contribute to the development of cancer. Moreover, the substance does not penetrate the skin, so the use of sunscreens that contain dioxide does not pose a danger, but a substance that enters the body - with food colorings, biological additives - can be dangerous.
Titanium is a uniquely strong, hard and lightweight metal with very interesting chemical and physical properties. This combination is so valuable that even the difficulties with smelting and purifying titanium do not stop manufacturers.
This video will tell you how to distinguish titanium from steel:
[edit] Durability
Drill coated with titanium nitride
In terms of specific strength, titanium has no rival among industrial metals. Even a metal such as aluminum has lost a number of positions to titanium, which is only 1.7 times heavier than aluminum, but six times stronger. And most importantly, titanium retains its strength at high temperatures (up to 500° C, and with the addition of alloying elements - 650° C), while the strength of most aluminum alloys drops sharply already at 300° C.
Titanium is a very hard metal: it is 12 times harder than aluminum, 4 times harder than iron and copper. The higher the yield strength of the metal, the better the parts made from it can withstand operational loads, the longer they retain their shapes and sizes. The yield limit of titanium is 18 times higher than that of aluminum, and 2.5 times higher than that of iron.
Chemical properties of titanium
Under normal conditions, an oxide film forms on the surface of this metal, which protects it from the destructive effects of moisture and acids. The chemical properties of titanium include its resistance to alkalis and chlorine solutions. Has an oxidation state of +4. It begins to interact with oxygen at a temperature of 600 degrees. Titanium filings may spontaneously ignite when heated.
Titanium crystal lattice:
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | α-titanium |
| 512 | Lattice structure | Hexagonal close-packed |
| 513 | Lattice parameters | a = 2.951 Å, c = 4.697 Å |
| 514 | c/a ratio | 1,587 |
| 515 | Debye temperature | 380 K |
| 516 | Name of space symmetry group | P63/mmc |
| 517 | Symmetry space group number | 194 |
| 521 | Crystal grid #2 | β-titanium |
| 522 | Lattice structure | Cubic body-centered |
| 523 | Lattice parameters | a = 3.269 Å |
| 524 | c/a ratio | |
| 525 | Debye temperature | |
| 526 | Name of space symmetry group | Im_ 3m |
| 527 | Symmetry space group number |
Isotopes
Known isotopes of titanium with mass numbers from 38 to 63 (number of protons 22, neutrons from 16 to 41), and 2 nuclear isomers.
Natural titanium consists of a mixture of five stable isotopes: 46Ti (isotopic abundance 7.95%), 47Ti (7.75%), 48Ti (73.45%), 49Ti (5.51%), 50Ti (5.34%) .
Among the artificial isotopes, the longest-lived are 44Ti (half-life 60 years) and 45Ti (half-life 184 minutes).
Physical properties
Titanium is a lightweight silvery-white metal. Exists in two crystal modifications: ?-Ti with a hexagonal close-packed lattice (a=2.951 A; c=4.697 A; z=2; space group C6mmc
), ?-Ti with cubic body-centered packing (a=3.269 A; z=2; space group
Im3m
), transition temperature ?-? 883 °C, ?H transition 3.8 kJ/mol. Melting point 1671 °C, boiling point 3260 °C, density of ?-Ti and ?-Ti, respectively, equal to 4.505 (20 °C) and 4.32 (900 °C) g/cm?, atomic density 5.71 × 1022 at /cm³. Plastic, weldable in an inert atmosphere.
It has a high viscosity and, during machining, is prone to sticking to the cutting tool, and therefore requires the application of special coatings to the tool and various lubricants.
At ordinary temperatures it is covered with a protective passivating film of TiO2 oxide, making it corrosion resistant in most environments (except alkaline).
Titanium dust tends to explode. Flash point 400°C.
Types of alloys
Titanium alloys can be divided into three large groups:
- Compounds based on chemical compounds. Representatives of this group have a heat-resistant structure and low density. A decrease in density directly affects a decrease in the weight of the material. Such alloys are used in the manufacture of parts for cars, frames for aircraft and hulls for ships.
- Heat-resistant alloys with low density. This is an analogue of compounds with nickel, but at a lower price. Depending on the chemical composition, the resistance of the titanium alloy to high temperatures varies.
- Structural - high-strength connections that are easy to process due to their high ductility. These alloys are used to make parts that are installed in equipment that operates under heavy loads.
When producing titanium alloys, official markings are used that indicate what metals it is combined with.
Physical properties
Main characteristics of titanium:
- temperatures: melting 1668 degrees Celsius, boiling – 3227;
- yield strength: from 250 to 380 MPa;
- elasticity – 110 GPa, varies in different directions;
- the average hardness of alloys according to HB is 103;
- density: at room temperature 4500 kg/m3, at melting point - 4120 kg/m3;
- heat capacity – 531 J per kilogram when heated by a degree;
- thermal conductivity – 18 W/(m deg);
- resistivity – 42.1·10-6 Ohm·cm.
When cooled to 3.8°K (-270°C), the metal becomes a superconductor.
Chemical properties
In the solid state, Ti is chemically stable and does not oxidize in high humidity, marine atmosphere, or in contact with aggressive environments. When heated to the melting point, it becomes active. Interacts with all air components:
- oxygen, solid oxides are formed;
- nitrogen, it strengthens the structure, increases the tensile strength, the critical concentration is 0.2%, above this indicator the metal becomes brittle;
- hydrogen degrades technological properties;
- carbon increases the temperature of phase changes.
When heated to the melting point, the metal must be insulated.
The dual properties of titanium metal
Many people are interested in the slightly mysterious and not fully studied titanium - a metal whose properties are somewhat ambiguous. Metal is both the strongest and most fragile.
The strongest and most fragile metal
It was discovered by two scientists with a difference of 6 years - the Englishman W. Gregor and the German M. Klaproth.
The name titan is associated, on the one hand, with the mythical titans, supernatural and fearless, and on the other hand, with Titania, the queen of fairies.
This is one of the most common materials in nature, but the process of obtaining pure metal is particularly complex.
Properties of titanium
22 chemical element of D. Mendeleev's table Titanium (Ti) belongs to group 4 of period 4.
The color of titanium is silver-white with a pronounced shine. Its glare shimmers with all the colors of the rainbow.
This is one of the refractory metals. It melts at a temperature of +1660 °C (±20°). Titanium is paramagnetic: it is not magnetized in a magnetic field and is not pushed out of it. The metal is characterized by low density and high strength.
But the peculiarity of this material is that even minimal impurities of other chemical elements radically change its properties.
In the presence of an insignificant proportion of other metals, titanium loses its heat resistance, and the minimum of non-metallic substances in its composition makes the alloy brittle.
This feature determines the presence of 2 types of material: pure and technical.
- Pure titanium is used where a very light substance that can withstand heavy loads and ultra-high temperature ranges is required.
- The technical material is used where parameters such as lightness, strength and corrosion resistance are valued.
The substance has the property of anisotropy. This means that the metal can change its physical characteristics based on the force applied. You should pay attention to this feature when planning the use of the material.
Titanium loses strength at the slightest presence of impurities of other metals
Studies of the properties of titanium under normal conditions confirm its inertness.
The substance does not react to elements in the surrounding atmosphere. Changes in parameters begin when the temperature rises to +400°C and above.
Titanium reacts with oxygen, can ignite in nitrogen, and absorbs gases.
These properties make it difficult to obtain a pure substance and its alloys. Titanium production is based on the use of expensive vacuum equipment.
Titanium and competition with other metals
This metal is constantly compared to aluminum and iron alloys. Many chemical properties of titanium are significantly better than those of competitors:
- In terms of mechanical strength, titanium is 2 times greater than iron, and aluminum 6 times. Its strength increases with decreasing temperature, which is not observed among competitors. The anti-corrosion characteristics of titanium significantly exceed those of other metals.
- At ambient temperatures the metal is completely inert. But when the temperature rises above +200°C, the substance begins to absorb hydrogen, changing its characteristics.
- At higher temperatures, titanium reacts with other chemical elements. It has a high specific strength, which is 2 times higher than the properties of the best iron alloys.
- The anti-corrosion properties of titanium significantly exceed those of aluminum and stainless steel.
- The substance does not conduct electricity well. Titanium has an electrical resistivity 5 times higher than that of iron, 20 times higher than that of aluminum, and 10 times higher than magnesium.
- Titanium is characterized by low thermal conductivity, this is due to its low coefficient of thermal expansion. It is 3 times less than that of iron, and 12 times less than that of aluminum.
How is titanium obtained?
The material ranks 10th in distribution in nature. There are about 70 minerals containing titanium in the form of titanic acid or titanium dioxide. The most common of them and containing a high percentage of metal derivatives are:
- ilmenite;
- rutile;
- anatase;
- perovskite;
- brookite.
The main deposits of titanium ores are located in the USA, Great Britain, Japan, large deposits have been discovered in Russia, Ukraine, Canada, France, Spain, and Belgium.
Titanium mining is an expensive and labor-intensive process
Extracting metal from them is very expensive. Scientists have developed 4 methods for producing titanium, each of which is functional and effectively used in industry:
- Magnesium-thermal method. The extracted raw materials containing titanium impurities are processed and titanium dioxide is obtained. This substance is subjected to chlorination in mine or salt chlorinators at elevated temperatures. The process is very slow and is carried out in the presence of a carbon catalyst. In this case, solid dioxide is converted into a gaseous substance - titanium tetrachloride. The resulting material is reduced with magnesium or sodium. The alloy formed during the reaction is heated in a vacuum unit to ultra-high temperatures. As a result of the reaction, magnesium and its compounds with chlorine evaporate. At the end of the process, a sponge-like material is obtained. It is melted and high quality titanium is obtained.
- Calcium hydride method. The ore is subjected to a chemical reaction to produce titanium hydride. The next stage is the separation of the substance into its components. Titanium and hydrogen are released during heating in vacuum units. At the end of the process, calcium oxide is obtained, which is washed with weak acids. The first two methods relate to industrial production. They make it possible to obtain pure titanium in the shortest possible time at relatively low costs.
- Electrolysis method. Titanium compounds are exposed to high current. Depending on the feedstock, compounds are divided into components: chlorine, oxygen and titanium.
- Iodide method or refining. Titanium dioxide obtained from minerals is doused with iodine vapor. As a result of the reaction, titanium iodide is formed, which is heated to a high temperature - +1300...+1400°C and is exposed to electric current. In this case, the following components are isolated from the source material: iodine and titanium. The metal obtained by this method has no impurities or additives.
Grades of titanium and alloys
The most common grades of titanium are VT1-0, VT1-00, VT1-00sv. Titanium of the indicated grades is called technical. These brands do not contain alloying elements, only a small amount of impurities. The Ti content in the VT1-0 grade is approximately 99.24-99.7%, in VT1-00 - 99.58-99.9%, VT1-00sv - 99.39-99.9%. VT1-0, VT1-00 are supplied in the form of sheets, plates, rods and pipes. The wire is most often used for various welding purposes and is produced from the VT1-00sv brand.
Currently, a fairly large number of serial titanium alloys are known, differing in chemical composition, mechanical and technological properties. The most common alloying elements in such materials are: aluminum, vanadium, molybdenum, manganese, chromium, silicon, tin, zirconium, iron.
Titanium alloy VT5 contains 5% aluminum. It has higher strength properties compared to titanium, but its manufacturability is low. The alloy is forged, rolled, stamped and welded well. Titanium rods (circles), wire and pipes, as well as sheets are produced from the VT5 grade. It is used in the manufacture of parts operating at temperatures up to 400 °C.
Titanium alloy VT5-1, in addition to 5% aluminum, contains 2-3% tin. Tin improves its technological properties. All types of semi-finished products obtained by pressure treatment are made from the VT5-1 grade: titanium plates, as well as sheets, forgings, stampings, profiles, pipes and wire. It is intended for the manufacture of products operating in a wide temperature range: from cryogenic (negative) to + 450 °C.
Titanium alloys OT4 and OT4-1 contain aluminum and manganese as alloying elements. They have high technological plasticity (they are well deformed in hot and cold states) and are well welded by all types of welding. This material is used mainly for the production of titanium plates and sheets, tapes and strips, as well as rods and circles, forgings, profiles and pipes. Titanium alloys OT4 and OT4-1 are used to produce parts using welding, stamping and bending, operating up to a temperature of 350 °C. These materials have disadvantages: 1) relatively low strength and heat resistance; 2) greater tendency to hydrogen embrittlement. In the PT3V alloy, manganese is replaced by vanadium.
Titanium alloy VT20 was developed as a more durable sheet material compared to VT5-1. The strengthening of the VT20 grade is due to its alloying, in addition to aluminum, with zirconium and small amounts of molybdenum and vanadium. The technological ductility of the VT20 alloy is low due to the high aluminum content, however, it has high heat resistance. This material welds well, the strength of the welded joint is equal to the strength of the base metal. The alloy is intended for the manufacture of products operating for a long time at temperatures up to 500 °C.
Titanium alloy VT3-1 belongs to the Ti – Al – Cr – Mo – Fe – Si system. It is usually isothermally annealed. This annealing provides the highest thermal stability and maximum ductility. The VT3-1 grade is one of the most widely used alloys in production. It is designed for long-term operation at 400 – 450 °C; it is a heat-resistant material with fairly high long-term strength. It is used to supply rods (titanium circles), profiles, plates, forgings, and stampings.
How to recognize titanium
Under normal everyday conditions, titanium can be distinguished from other metals using several methods.
Mathematical method
It is not difficult to effectively apply a mathematical approach to titanium recognition when there are several samples of different metals. This method is based on the weight of the product that needs identification.
It should be noted that the density indicator for visually similar metals is significantly different.
The density indicator is:
- titanium 4.5;
- duralumin and aluminum 2.7;
- iron 7.8.
The density of steel is affected by the composition of a particular grade, but this difference is not significant when using this method. Therefore, in practice it is generally accepted that it is equal to the iron indicator. Weighing items of the same volume made from different materials will reveal a titanium sample.
To identify a titanium product in the absence of aluminum or steel samples for comparison, simple mathematical calculations will help, for which it is necessary to determine the volume and weight of the sample under study. For products with complex configurations, Archimedes' law will help determine the volume. The volume of water that the product will push out when immersed in the container will be equal to the volume of this product itself. It is convenient to use a container with a division scale printed on it. Taking into account the density of water, one gram of displaced liquid is equal to a cubic centimeter of volume.
Marks on tiles or glass
A very simple and accessible method allows you to easily find out whether an object in the hands of a researcher is titanium. It is noteworthy that titanium leaves virtually indelible patterns on tiles or glass surfaces.
You will need to draw the pointed edge of the sample over the surface of one of the specified materials. Titanium will not leave scratches, but marks that are not easy to wash off. This is the method used to apply graphic images to bus windows. It allows titanium to be tested efficiently and easily. An aluminum or steel sample can only slightly scratch the surface. To conduct the experiment, there is no need to clean and degrease the glass or tiled surface.
Application of abrasive
A tool sharpening machine or any other abrasive material (even asphalt pavement on the road) will help you easily distinguish titanium from stainless steel. If, upon contact of the test sample with an abrasive, a fountain of bright white sparks appears, then it is definitely titanium. Treating the steel surface will produce a minimal amount of reddish or yellowish sparks.
Stainless steel has a high level of fire safety and therefore practically does not produce sparks. This property allows the use of stainless steel tools in conditions of high fire danger.
It is noteworthy that the impact of abrasive on aluminum parts is not accompanied by sparks.
Thus, this is the most accessible and effective method.
Galvanic method
It is easy to use the technique in a workshop or garage. This titanium identification technology is based on its ability to change color during the anodizing process.
For the study, you will need a regular car battery, with a titanium plate connected to the positive terminal. A steel rod wrapped in cotton wool is connected to the “minus” of the energy source. The cotton wool must be soaked in Coca-Cola or any saline solution.
When processing titanium with cotton wool, it will change its color in a few seconds. The shade resulting from the appearance of an oxide film is determined by the contact period and operating voltage. The color is not of fundamental importance, the fact of coloring is important.
Specific methods for titanium identification
In practice, several specific methods are used to distinguish titanium from aluminum, magnesium or stainless steel.
- Having received thin shavings from the analyzed sample, it must be set on fire. Titanium shavings immediately ignite and burn brightly, while aluminum shavings only melt.
- By placing duralumin shavings in an alkali solution, it is easy to notice the rapid release of hydrogen.
- Titanium and its alloys have low thermal conductivity. Therefore, when one edge of the part is heated, the opposite edge will remain cold.
- Low thermal conductivity gives the impression of warm metal in the hands, as opposed to cooling aluminum or steel. But in the case of stainless steel, this method is not recommended, since it will also feel warm to the touch.
- When you hit the test sample with a hammer, there will be no marks left on the steel surface, a small dent will appear on the titanium surface, and a noticeable defect will form on the aluminum surface.
It is important to understand that the composition of the alloy directly determines its technical properties. Therefore, when applied to some multicomponent materials, household metal identification methods can produce erroneous results. The most accurate method is to analyze the chemical composition of the sample under study using specialized equipment.
Advantages/disadvantages
- Advantages:
- low density (4500 kg/m3) helps reduce the weight of manufactured products;
- high mechanical strength. It is worth noting that at elevated temperatures (250-500 °C) titanium alloys are superior in strength to high-strength aluminum and magnesium alloys;
- unusually high corrosion resistance due to the ability of Ti to form thin (5-15 μm) continuous films of TiO2 oxide on the surface, firmly associated with the mass of the metal;
- the specific strength (the ratio of strength and density) of the best titanium alloys reaches 30-35 or more, which is almost twice the specific strength of alloy steels.
- Flaws:
- high production cost, Ti is much more expensive than iron, aluminum, copper, magnesium;
- active interaction at high temperatures, especially in the liquid state, with all gases that make up the atmosphere, as a result of which Ti and its alloys can only be melted in a vacuum or in an environment of inert gases;
- difficulties in involving titanium waste into production;
- poor antifriction properties due to Ti adhesion to many materials; titanium paired with titanium cannot work on friction at all;
- high susceptibility of Ti and many of its alloys to hydrogen embrittlement and salt corrosion;
- poor machinability, similar to the machinability of austenitic stainless steels;
- high chemical activity, tendency to grain growth at high temperatures and phase transformations during the welding cycle cause difficulties in welding titanium.
[edit] Application
Titanium and its alloys with Al, V, Mo, Mn, Cr, Si, Fe, Sn, Zr, Nb, Ta are used as a structural metal in aviation and rocket technology, shipbuilding, power engineering, food, medical industries and non-ferrous metallurgy, where they are used reliably and for a long time in many chemically aggressive environments. The most important are titanium-vanadium alloys, which have high strength, ductility and weldability; Titanium carbide is used for the production of super-hard alloys, titanium dioxide is used for the production of stable titanium white, plastics and in the pulp and paper industry; Titanium oxide TiO has metallic conductivity and is used in electrochromic systems.
Titanium is one of the few metals with high corrosion resistance: it is stable in atmospheric air, sea water and marine atmosphere, in wet chlorine, hot and cold chloride solutions, in various technological solutions and reagents used in the chemical, oil, paper and other industries industry, as well as in hydrometallurgy.
In terms of its corrosion resistance in sea water, it surpasses all metals, with the exception of noble ones - gold, platinum, etc., most types of stainless steel, nickel, copper and other alloys. The fact is that reactions of titanium with many elements occur only at high temperatures. At ordinary temperatures, the chemical activity of titanium is extremely low and it practically does not react. This is due to the fact that on a fresh surface of pure titanium, as soon as it is formed, an inert, thin (several angstrom film of titanium dioxide (passivation)) appears very quickly, which grows well with the metal, which protects it from further oxidation. Even if this film removed, then in any environment containing oxygen or other strong oxidizing agents (for example, in nitric or chromic acid), this film appears again, and the metal is said to be passivated, that is, it protects itself from further destruction.
Properties and applications of titanium alloys
Titanium alloys do not have the main disadvantages of pure metal. When adding third-party materials, its characteristics change. Key properties of titanium alloys:
- resistance to corrosion processes;
- low density;
- high specific strength.
Alloys are also more resistant to high temperatures. Thanks to increased protection against acids and alkalis, alloys based on this material have gained popularity in the chemical industry and medicine. They are used in construction, production of equipment, cars, airplanes, rockets and ships.
Titanium and compounds based on it are common in various industries. This metal has unique characteristics that set it apart from other materials. Due to the difficulties of obtaining pure metal, its price is quite high.
In pure form and in the form of alloys
Titanium alloy watch F-15 fighter titanium frame blank before and after pressing on Alcoa's 45,000 ton stamping press, May 1985
The use of titanium metal in many industries is due to the fact that its strength is approximately equal to that of steel while being 45% lighter. Titanium is 60% heavier than aluminum, but is about twice as strong.
- Titanium in the form of alloys is the most important structural material in aircraft, rocket and shipbuilding.
- The metal is used in the chemical industry (reactors, pipelines, pumps, pipeline fittings), military industry (body armor, aviation armor and fire barriers, submarine hulls), industrial processes (desalination plants, pulp and paper processes), automotive industry, agricultural industry , food industry, sporting goods, jewelry, mobile phones, light alloys, etc.
- Titanium is physiologically inert, which is why it is used in medicine (prostheses, osteoprostheses, dental implants), in dental and endodontic instruments, and piercing jewelry.
- Titanium casting is performed in vacuum furnaces into graphite molds. Vacuum lost wax casting is also used. Due to technological difficulties, it is used in artistic casting to a limited extent. The first monumental cast titanium sculpture in the world is the monument to Yuri Gagarin on the square named after him in Moscow.
- Titanium is an alloying additive in many alloy steels and most special alloys.
- Nitinol (nickel-titanium) is a shape memory alloy used in medicine and technology.
- Titanium aluminides are very resistant to oxidation and heat-resistant, which, in turn, determined their use in aviation and automotive manufacturing as structural materials.
- Titanium is one of the most common getter materials used in high-vacuum pumps.
There are many titanium alloys with different metals. Alloying elements are divided into three groups, depending on their effect on the temperature of the polymorphic transformation: beta stabilizers, alpha stabilizers and neutral strengtheners. The first ones lower the transformation temperature, the second ones increase it, the third ones do not affect it, but lead to solution strengthening of the matrix. Examples of alpha stabilizers: aluminum, oxygen, carbon, nitrogen. Beta stabilizers: molybdenum, vanadium, iron, chromium, nickel. Neutral hardeners: zirconium, tin, silicon. Beta stabilizers, in turn, are divided into beta isomorphic and beta eutectoid-forming.
The most common titanium alloy is the Ti-6Al-4V alloy (VT6 in the Russian classification), containing about 6% aluminum and about 4% vanadium. Based on the ratio of crystalline phases, it is classified as an (α+β) alloy. Up to 50% of mined titanium is used for its production.
Ferrotitanium (an alloy of titanium with iron containing 18-25% titanium) is used in ferrous metallurgy to deoxidize steel and remove unwanted impurities dissolved in it (sulfur, nitrogen, oxygen).
In the 1980s, about 60-65% of titanium mined in the world was used in the construction of aircraft and rockets, 15% in chemical engineering, 10% in energy, 8% in shipbuilding and for water desalination plants.
In the form of connections
- White titanium dioxide (TiO2) is used in paints (such as titanium white) and in the production of paper and plastics. Food additive E171.
- Organo-titanium compounds (for example, tetrabutoxytitanium) are used as a catalyst and hardener in the chemical and paint and varnish industries.
- Inorganic titanium compounds are used in the chemical electronics and fiberglass industries as additives or coatings.
- Titanium carbide, titanium diboride, and titanium carbonitride are important components of superhard materials for metal processing.
- Titanium nitride is used to coat instruments, church domes and in the production of costume jewelry, as it has a color similar to gold.
- Barium titanate BaTiO3, lead titanate PbTiO3 and a number of other titanates are ferroelectrics.
- Titanium tetrachloride is used to iridize glass and to create smoke screens.
Properties of titanium atom:
| 200 | Properties of the atom | |
| 201 | Atomic mass (molar mass) | 47.867(1) amu (g/mol) |
| 202 | Electronic configuration | 1s2 2s2 2p6 3s2 3p6 3d2 4s2 |
| 203 | Electronic shell | K2 L8 M10 N2 O0 P0 Q0 R0 |
| 204 | Atomic radius (calculated)* | 176 pm |
| 205 | Empirical atomic radius* | 140 pm |
| 206 | Covalent radius* | 160 pm |
| 207 | Ion radius (crystalline) | Ti2+ 100 (6) pm, Ti3+ 81 (6) pm, Ti4+ 56 (4) pm, 74.5 (6) pm (in parentheses the coordination number is indicated - a characteristic that determines the number of nearest particles (ions or atoms) in a molecule or crystal) |
| 208 | Van der Waals radius | |
| 209 | Electrons, Protons, Neutrons | 22 electrons, 22 protons, 26 neutrons |
| 210 | Family (block) | d-family element |
| 211 | Period in the periodic table | 4 |
| 212 | Group on the periodic table | 4th group (according to the old classification - a secondary subgroup of the 4th group) |
| 213 | Emission spectrum |
Pros and cons of metal and its alloys
Advantages of titanium alloys:
- The strength-density ratio of titanium alloys is almost 2 times better than that of alloy steels.
- High mechanical strength.
- Excellent corrosion resistance, which allows the products to work in aggressive environments.
Titanium alloy watches
The disadvantages of titanium alloys include:
- High price (titanium is much more expensive than many non-ferrous metals).
- When processing metal and its alloys, the problem of sticking arises, which threatens rapid wear of cutting tools.
- Difficulties in welding titanium products.
[edit] Titanium is an anti-corrosion metal
[edit] Behavior of titanium and its alloys in various aggressive environments
Reactions of titanium with many elements occur only at high temperatures. At ordinary temperatures, the chemical activity of titanium is extremely low and it practically does not react. This is due to the fact that on the fresh surface of pure titanium, as soon as it is formed, an inert film very quickly appears, such that the thinnest (several angstroms (1A = 10−10 m) film of titanium dioxide grows well with the metal, which protects it from further oxidation. Even if this film is removed, then in any environment containing oxygen or other strong oxidizing agents (for example, in nitric or chromic acid), this film appears again, and the metal, as they say, is “passivated” by it, that is, it protects itself yourself from further destruction.
[edit] The influence of alloying elements in titanium on corrosion resistance
All alloying elements present in titanium can be divided into four groups based on corrosion resistance. The first group includes elements that are easily passivated and increase the corrosion resistance of titanium by inhibiting the anodic process (to varying degrees and depending on the nature of the medium). This group includes: Mo, TaNb, Zr, V (arranged in descending order of beneficial effect on corrosion resistance).
The second group of metals that have a similar effect on the corrosion resistance of titanium includes Cr, Ni, Mn, Fe. These elements, some of which are themselves corrosive (Cr, Ni), although not greatly, reduce the corrosion resistance of titanium, especially in non-oxidizing acids as titanium alloying increases.
The third group of alloying elements that have a common effect on the corrosion resistance of titanium includes Al, Sn, C. It has been established that aluminum additives reduce the corrosion resistance of titanium in the active and passive states. In neutral environments, aluminum (up to 5% Al), although it has a negative effect, is small. The decrease in corrosion resistance when alloying with aluminum is associated with the facilitation of the anodic and cathodic processes due to changes in the chemical nature of passive films.
The fourth group of alloying elements, which have the same effect on the corrosion resistance of titanium, includes metals with low cathodic resistance. In order of increasing effectiveness of their impact on titanium, these elements are arranged in the following row: CuW, MoNi, Re, Ru, Pd, Pt.
It has been proven that the introduction of elements such as molybdenum, niobium, zirconium, tantalum into titanium alloys is not limited in quantity. They increase corrosion resistance and help increase strength.
[edit] Features of the interaction of titanium with air
Air, which is a mixture of various gases, is a complex gas phase, the effect of which on titanium can be very diverse. In this case, the interaction of titanium with oxygen in the air differs from the interaction of titanium with pure oxygen, since this interaction is influenced by nitrogen and other components of the air. At the same time, it should be borne in mind that with all the complexity of the gas phase (air), its effect on titanium should be considered primarily as a reaction of interaction with it of an active and quite significant component - oxygen.
Titanium casting
During heating to the melting temperature, titanium actively reacts with air components.
To prevent this from happening, the air in the furnaces was pumped out and a vacuum was created. The remaining air began to be replaced by inert gases: a mixture of argon and helium. In industrial foundry installations, the residual pressure of inert gases ranges from 1.33 to 0.13 Pa.
Several technologies have been developed:
In a vacuum chamber, the metal is melted and poured into molds. It is cooled to a temperature when the metal loses its chemical activity and forms a crystalline structure.
The method of vacuum casting (MVL) using lost wax models involves the use of lost wax or burnt out molds. A fire-resistant shell is created on the surface of the model. The castings are obtained in the most approximate shape.
Shell casting technology involves the use of thin-walled split molds. They are placed on a heated model plate to be coated with thermoactive resin. Filling is done vertically and horizontally.
The temperature regime for cooling the castings is specially developed. Uniform structuring is provided throughout the entire volume so that internal stresses do not arise in the casting.
Interesting Facts
There are a lot of interesting facts related to titanium. It’s worth starting with the fact that the gong (musical instrument) is made of pure titanium. Pipes made for pumping oil and gas are made from titanium due to its strength and corrosion resistance. Another interesting point is that titanium in powder form is flammable. Compounds based on titanium powder are used in pyrotechnics. Titanium is also used as an electrode in lithium and lithium-ion batteries.
Method of obtaining from raw materials
The starting raw material is titanium dioxide, which contains few foreign impurities.
To do this, you need a rutile concentrate obtained by ore enrichment. But its global reserves are small, and titanium slag (synthetic rutile) is more often used, which is obtained by heat treatment - enrichment of ilmenite concentrates in an electric arc furnace. As a result, iron in the form of cast iron is collected at the bottom of a special bath, and a gray powder remains - slag containing titanium oxide. It is crushed, mixed with coal, briquetted and chlorinated in furnaces, where titanium tetrachloride vapors are formed at 800 °C in the presence of carbon. Then they are purified and reduced with magnesium in special reactors at 950 °C. A sintered porous mass, a titanium sponge, is formed on the walls, which is calcined in a vacuum to separate it from magnesium compounds. To produce titanium ingots, the resulting sponge is melted in vacuum arc furnaces. This protects the metal from oxidation and contributes to the final release of impurities. Finished ingots with a purity of up to 99.7% are used for pressure processing (rolling, stamping, forging).
Sources
- https://metalloy.ru/splavy/titan-i-ego-splavy
- https://tokar.guru/metally/unikalnye-svoystva-metalla-titan-plotnost-i-temperatura-plavleniya.html
- https://chem.ru/titan.html
- https://stroyres.net/metallicheskie/vidyi/tsvetnyie/titan/fizicheskie-harakteristiki-i-svoystva.html
- https://ChemicalStudy.ru/titan-svoystva-atoma-himicheskie-i-fizicheskie-svoystva/
- https://svarkaprosto.ru/tehnologii/temperatura-plavleniya-titana
- https://www.metotech.ru/titan-opisanie.htm
- https://TheMineral.ru/metally/titan
Receipt
A block of crystalline titanium (purity 99.995%, weight ? 283 g, length ? 14 cm, diameter ? 25 mm), manufactured using the van Arkel and de Boer iodide method. The
titanium ore concentrate is subjected to sulfuric acid or pyrometallurgical processing. The product of sulfuric acid treatment is titanium dioxide powder TiO2. Using the pyrometallurgical method, the ore is sintered with coke and treated with chlorine, producing titanium tetrachloride vapor TiCl4: TiO2 + 2C + 2Cl2 = TiCl4 + 2CO
The resulting TiCl4 vapors at 850 °C reduce Mg: TiCl4+ 2Mg = 2MgCl2+ Ti
The resulting titanium “sponge” is melted down and cleaned. Ilmenite concentrates are reduced in electric arc furnaces, followed by chlorination of the resulting titanium slag. Titanium is refined using the iodide method or electrolysis, separating Ti from TiCl4. To obtain titanium ingots, arc, electron beam or plasma processing is used.
STRUCTURE
Crystal crystal structure
Titanium has two allotropic modifications. The low-temperature modification, existing up to 882 °C, has a hexagonal close-packed lattice with periods a = 0.296 nm and c = 0.472 nm. The high-temperature modification has a body-centered cube lattice with a period a = 0.332 nm. The polymorphic transformation (882 °C) with slow cooling occurs according to the normal mechanism with the formation of equiaxed grains, and with rapid cooling - according to the martensitic mechanism with the formation of an acicular structure. Titanium has high corrosion and chemical resistance due to the protective oxide film on its surface. It does not corrode in fresh and sea water, mineral acids, aqua regia, etc.