Definition and decoding
Critical points are temperature values that result in changes in the physical and chemical properties of the steel alloy.
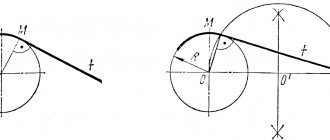
Such indicators are designated by the letter A. At the lower value of the point on the graph on the PSK straight line, the steel has the “iron-carbon” state. This point is called A1, it is at this value that austenite transforms into pearlite during the cooling of the material, and pearlite into austenite when the alloy is heated. At the top of the diagram, the critical value is the Az value. For hypoeutectoid steel alloys, Az is on the straight line GS. At this value, ferrite is released during the cooling process of iron and its dissolution stops when heated. Point Az for hypereutectoid steel alloys is located on straight line SE. At this value, secondary cementite is released during the cooling of the steel material, and dissolution stops when the temperature rises.
The designation of critical points during heating differs from critical points of temperature reduction by a small letter “c” and the letter “r”, respectively, since “c” means “chauffage” (translated from French as heating), and “r” means “refroidissement” ( translation – cooling). The abbreviation also contains a digital value characterizing these changes.
Thus, Ar1 denotes the point at which austenite turns into pearlite, and at point Ac1, on the contrary, pearlite turns into austenite. The Ar3 value corresponds to the process of ferrite precipitation into austenite, and the end of ferrite dissolution in austenite is the Ac3 point. Ar3 also means the beginning of the process of separating cementite from austenite, and the Ac3 point means the end of dissolution. In addition, when a hypoeutectoid alloy is heated above the value of the GS line (Point A3) it is often designated as point A cm.
The abbreviation Mn is the point at which martensitic transformation begins on the graph.
What are critical points of steel
Critical points of steel or Chernov points are critical temperatures at which a change in the phase state and structure of steel occurs when it is heated or cooled in solid form. Installed by Dmitry Konstantinovich Chernov in 1868.
Critical points are designated by the letter A. The lower critical point corresponds to the PSK line of the iron-carbon phase diagram. This point is called A1 and corresponds to the transformation of austenite into pearlite when cooled or pearlite into austenite when heated. The upper critical point is called A3. Critical point A3 for hypoeutectoid steels lies on the GS line of the iron-carbon diagram and corresponds to the beginning of ferrite precipitation upon cooling or the end of its dissolution upon heating. Critical point A3 for hypereutectoid steels lies on the SE line and corresponds to the beginning of the release of secondary cementite during cooling or the end of its dissolution during heating.
Depending on whether the critical point is determined during heating or cooling, the index “c” is added to the letter A when heating (from the French word chauffage - heating) and the index "r" (from the French word refroidissement - cooling) when cooling, leaving the number characterizing this transformation.
Thus, for example, heating of hypoeutectoid steel above the corresponding point on the GS line is designated as heating above point Ac3 . When cooling this steel, the first transformation should be designated as Ar3 , the second (on the RSK line) - as Ar1 . Point A3 for hypereutectoid steels is usually designated Аcm .
The point Mn in the table indicates the temperature at which the martensitic transformation begins.
When heat treating steels, critical point values are most often used to determine the heating temperature for hardening.
Changes in the state of steel at critical points
Critical points during heat treatments are usually necessary to determine the temperature conditions required for hardening steel.
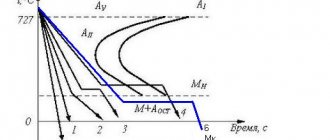
The line to tn is the values during heating from the initial cooled state of point to to melting at point tn. If a soft steel alloy, which is in a cooled state and does not form cracks when bent and unbent, is increased to the temperature t1 and subjected to bending, then at this temperature it can lose its toughness and may break. This temperature is the temperature of blue tarnish (or steel tarnish), equal to approximately 375 0C, denoted by the letter K, this state of steel is called blue brittleness. If heating continues further, and the temperature becomes higher than t1, then the material will again become viscous and flexible, and the part will gradually elongate, and its temperature will rise correctly. With further heating no more than temperature t2, if the material is quickly cooled, it will not accept hardening at all.
As soon as the temperature rises to the value of point t2, the steel stops elongating, the temperature does not increase and even begins to gradually decrease. If you slowly cool a steel part that was heated no higher than the temperature of point t2, the bar, in the temperature regime at point t2, stops shortening, and its temperature not only does not decrease, but, no matter what, begins to rise and the steel becomes lighter. This critical point may vary depending on the hardness of the steel. The temperature of the critical points varies from 580 to 680 degrees, and this phenomenon is called recalescence, self-heating or glare. At these critical points, the steel alloy undergoes chemical changes. When heating a steel material with carbide at point t2, decomposition occurs with the release of carbon and dissolution in the iron mass. If the material is slowly cooled, then at t2 carbon and steel combine into carbide. In this case, in the first case, processes associated with the absorption of heat occur, and in the second, its release.
The critical point t2 was noticed in the seventies by the scientist D.K. Chernov, who proposed to determine its value in the following form: steel material, when heated below this value, is not able to harden. Critical points are temperature regimes that can change the phase states and structure of the alloy by heating or cooling the part. If you continue to heat the steel material, but not above the temperature value t3, the steel, despite the fact that it is capable of accepting hardening, will not change its structure with a slow or rapid decrease in temperature, and will remain the same as it was before heating. As the temperature rises to Chernoff values with temperature t3, rapid rearrangement occurs as the cooled material changes its structural state from coarse to fine-grained. This temperature makes it possible to ensure that the softened grains of the steel alloy are firmly connected and transform into a waxy material with an amorphous composition, which does not change when the temperature drops below the t3 value. Slow cooling of a part heated above the temperature regime of point t3 again causes the steel material to disintegrate into grains, and crystallization itself depends on a further change in the temperature regime further from point t3 to the right and on the time interval that passed before it decreased to the critical value t3. This crystallization process can be stopped by rapidly cooling the part to a temperature of critical points below t3, that is, to points where the crystallization process no longer occurs. Thus, the critical points at the value of t3 indicate the boundary at which the crystallization process ends when the hot steel alloy is cooled. This critical point ranges from 700 to 800 degrees Celsius, depending on the composition of the steel alloy and the carbon particle content.
In addition to all the phenomena listed above, according to the new results of Osmond’s research, at such a point the transformation of steel from one state to another occurs.
Thanks to research in the field of melting alloys, it was discovered that in an unquenched or well-annealed steel alloy, the iron material has a soft state, while in a hardened one it is hard. When heating an unhardened alloy, the metal changes state at t3, and heat is absorbed. If you slowly cool a hot part to critical points above the t3 value, at such values the alloy changes its state, releasing heat.
Such a change requires some time, in the absence of which it either makes no sense to transition to another state, or such a transition is not completed completely. Thus, when cooled quickly, the steel becomes harder, and when cooled slowly, the steel alloy becomes soft. Critical points are not constant and depend on the hardness of the alloy. The steel material becomes even softer as it moves away from point t0. In this case, the value of the critical points depends on the type of steel material, which is transformed at temperatures from 700 to 855 degrees Celsius.
Calculation of critical points of steels
I thought for a long time about the introduction to this article... the muse never visited me, so the article will be short and to the point. I am posting formulas for calculating the critical points A1, A3 and Ac1, Ac3!
Table
| Temperature, °C | Notes |
| A1=723-16.9 Ni + 29.1 Si + 6.38 W — 10.7 Mn + 16.9 Cr + 290 As A3 = 910 — 203√С + 44.7 Si — 15.2 Ni + 31.5 Mo + 104 V + 13.1 W — 30.0 Mn + 11.0 Cr + 20.0 Cu – 700 P – 400 Al – 120 As – 400 Ti | Calculation of temperatures of points corresponding to the equilibrium state. Both formulas are valid for low alloy steels with a carbon content of up to 0.6%. Source - [1]. |
| A1 = 712-17.8 Mn - 19.1 Ni + 20.1 Si + 11.9 Cr + 9.8 Mo A3 = 871- 254.4 √C - 14.2 Ni + 51.7 Si | Calculation of temperatures of points corresponding to the equilibrium state. Both formulas are valid for low alloy steels with a carbon content of up to 0.6%. Source - [2]. |
| A1 = 5/9 (1333 - 25 Mn + 40 Si + 42 Cr - 26 Ni - 32) A3 = 5/9 (1570 - 323 C - 25 Mn + 80 Si - 3 Cr -32 Ni - 32) | Calculation of temperatures of points corresponding to the equilibrium state. The formula is given taking into account the conversion of degrees °F to degrees °C: t[°C] = 5/9 (t[°F] - 32). In source [3], the formula is given in °F. |
| Ac1 = 739 - 22 C - 7 Mn + 2 Si + 14 Cr + 13Mo - 13 Ni Ac3 = 902 - 255 C - 11 Mn + 19 Si - 5 Cr + 13 Mo - 20 Ni + 55V | Temperatures of transformation of ferrite into austenite when heated. Source - [4]. |
| Ac1 = 754.83 – 32.25 C – 17.76 Mn + 23.32 Si + 17.3 Cr + 4.51Mo + 15.62 V | Source - [5]. |
| Ac1 = 723 - 7.08 Mn + 37.7 Si + 18.1 Cr + 44.2 Mo + 8.95 Ni + 50.1 V + 21.7 Al + 3.18 W + 297 S - 830 N - 11.5 CSi - 14.0 MnSi - 3.10 SiCr - 57.9 CMo - 15.5 MnMo - 5.28 CNi - 6.0 MnNi + 6.77 SiNi - 0.80 CrNi + 27.4 CV + 30.8 MoV - 0.84Cr² - 3.46 Mo² - 0.46 Ni² - 28 V² Ac3 = 912 - 370 C - 27.4 Mn + 27.3 Si - 6.35 Cr - 32.7 Ni + 95.2 V + 190 Ti + 72.0 Al + 64.5 Nb + 5.57 W + 332 S + 276 P + 485 N — 900 B + 16.2 CMn + 32.3 CSi + 15.4 CCr + 48.0 CNi + 4.32 SiCr — 17.3 SiMo — 18.6 SiNi + 4.80 MnNi + 40.5 MoV + 174 C² + 2.46 Mn² – 6.86 Si² + 0.322 Cr² + 9.90 Mo² + 1.24 Ni² + 60.2V² | Formulas are obtained for the following range of changes in the content of chemical elements: C ≤ 0.83%, Mn ≤ 2.0%, Si ≤ 1.0%, Cr ≤ 2.0%, Mo ≤ 1.0%, Ni ≤ 3.0%, V ≤ 0.5%, W ≤ 1.0%, Ti ≤ 0.15%, Al ≤ 0.2%, Cu ≤ 1.0%, Nb ≤ 0.20%, P ≤ 0.040%, S≤ 0.040%, N ≤ 0.025%, B ≤ 0.010%. Source - [6]. |
| Ac1 = 739.3 − 22.8 C − 6.8 Mn + 18.2 Si + 11.7 Cr −15 Ni − 6.4 Mo − 5 V − 28 Cu Ac3 = 937.3 − 224.5 C0.5 − 17 Mn + 34 Si − 14 Ni+21.6 Mo + 41.8 V − 20 Cu | The formula was obtained for steels with C = 0.11-0.77, Mn = 0.2-1.53, Si 0.14-1.37, Cr ≤ 1.54, Ni ≤ 1.72, Mo ≤ 0, 72, V ≤ 0.31, Cu ≤ 0.26 |
I have laid out the formulas for calculating temperatures A3 and Ac3 in the form of an Excel document. So you can download by following the link and use at your own peril and risk.