Deoxidation of steel [edit | edit code ]
Steel is usually deoxidized with aluminum, which forms a very strong oxide Al2O3, which is released in the liquid metal as a separate solid phase. Carbon, ferrosilicon, ferromanganese, silicocalcium [1] and silicomanganese [1] are also used.
Methods for steel deoxidation [edit | edit code ]
The following methods of steel deoxidation are common [2]:
- Precipitation deoxidation
- Diffusion deoxidation
- Treatment with synthetic slags
- Electroslag remelting
- Vacuum deoxidation
Precipitation deoxidation
- deoxidation, which uses elements that have a greater affinity for oxygen than iron. The most common deoxidants in precipitating deoxidation are silicon, aluminum, and manganese. Complex deoxidizers are also used.
Diffusion deoxidation
(
extraction deoxidation
) is a process in which a decrease in the oxygen content in steel occurs due to the deoxidation of slag. Aluminum, carbon, and silicon are usually used as deoxidizers in this deoxidation method.
Treatment with synthetic slags
— deoxidation of steel in an arc furnace by treating it with slag consisting of CaO and Al2O3. This deoxidation method is used to reduce the sulfur and oxygen content in steel.
Electroslag remelting
(
ESH
) is a method of steel deoxidation in which the alloy is subjected to melting in a slag bath. This method allows you to clean steel from non-metallic inclusions, such as sulfur.
Vacuum carbon deoxidation
— the process of purifying steel from oxygen in a vacuum, since under these conditions the deoxidizing properties of carbon are much more pronounced.
Menu
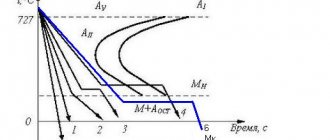
Romashkin A.N.After the oxidation period, an unacceptably large amount of oxygen is dissolved in the steel, depending mainly on the concentration of carbon and significantly exceeding the equilibrium value with it (determined from the ratio [C]∙[O] = 0.0025%). If this content is maintained until the period of crystallization, then, due to a decrease in the solubility of the elements in question, their intense interaction will occur and, as a consequence, the release of gaseous CO and CO2 (boiling effect). Such gas evolution and, accordingly, the presence of gas cavities in the metal is permissible only in a small number of cases (see the area of application of boiling and semi-quiet steels GOST 380-88 - Structural carbon steel of ordinary quality), as a rule, it is necessary to provide metal with minimal disruption of continuity, t .e. the occurrence of a carbon oxidation reaction during solidification must be excluded. In addition, even when producing boiling or semi-calm steel, the boiling intensity must be quite certain, allowing one to obtain a normal ingot. Therefore, in liquid steel it is always necessary to reduce the oxygen content to a level that ensures the required behavior of the metal during crystallization, which, in fact, is the purpose of deoxidation.
Deoxidation of boiling steel is reduced only to a slight decrease in the oxygen content in the metal while maintaining its level above the equilibrium level with carbon. This is usually ensured by deoxidation only with manganese with a residual content of 0.3...0.4% Mn; silicon is rarely additionally introduced (residual content no more than 0.02...0.03% Si) and/or aluminum (thousandths of a percent). Deoxidation of semi-quiet steel means obtaining a residual oxygen content in the metal slightly lower (usually by about 10...15% relative) than the equilibrium one. Only when this condition is met does the semi-quiet steel ingot form normally: the carbon oxidation reaction proceeds only to the extent that it is necessary to fill the shrinkage voids with gases and form a healthy outer crust of equiaxed crystals. Obtaining such a residual oxygen content is not an easy task, since slight excessive or insufficient deoxidation leads to disruption of the normal course of crystallization of the ingot. In most cases, when deoxidizing semi-mild steel, in addition to the usual manganese content (0.4...0.5%), it is enough to have 0.08...0.12% Si or a few thousandths of a percent of aluminum in the final metal.
Deoxidation of calm steel can be considered normal if the residual oxygen content is significantly lower than the equilibrium content with carbon; for this it is enough to have a residual content in the finished metal of 0.3...0.5% Mn and 0.2...0.3% Si.
It should be borne in mind that at a high oxygen content (at the level of equilibrium with carbon or more), the harmful effect of the non-metallic phase increases significantly; A manifestation of this is, for example:
- increase in general contamination with non-metallic inclusions;
- increase in the size of inclusions;
- release of film sulfides.
Therefore, to ensure the highest properties of metal products, the minimum possible oxygen content in steel should be ensured. The main way to achieve this is the so-called “deep” or “precipitating” deoxidation, which consists in converting dissolved oxygen into an insoluble oxide by introducing a deoxidizer - an element with a high affinity for oxygen - into the metal volume. In this case, as a rule, they strive to use complex deoxidation, which consists of simultaneously introducing several elements with a high affinity for oxygen. The effect of this technological technique is to reduce the activity of deoxidation products due to their interaction with each other. In addition, the complex oxides formed in this case have a lower melting point, the rate of their enlargement increases, and, accordingly, the conditions for removing the resulting deoxidation products are improved.
It is desirable to include in the composition of complex deoxidizers such components that, upon oxidation, form inclusions with minimal surface interfacial tension at the metal-inclusion boundary, which greatly facilitates the process of isolating non-metallic inclusions (such components, first of all, include manganese).
In addition to deep deoxidation, diffusion deoxidation is also used, which consists in the deoxidation of slag. The method is based on the fact that, in cases where there is no boiling of the bath between the activity of oxygen in the metal and the slag, there is a certain ratio aFeO/aO = const (the law of distribution of impurities dissolved in two immiscible liquids). Accordingly, any method of reducing the activity of iron oxides in the slag leads to a decrease in the oxidation of the metal. Usually, with this method of deoxidation, mixtures are given to the slag, which include strong reducing agents: carbon (coke, charcoal, electrode waste), silicon, aluminum chaff, powders of calcium-containing materials (silicocalcium, calcium carbide), etc.
A characteristic feature of this deoxidation method is that deoxidation reactions do not occur in the metal itself and, accordingly, oxygen compounds are not formed. The consequence of this is greater purity of the steel in terms of primary and secondary endogenous inclusions. However, this method is very time-consuming and practically does not allow obtaining deeply deoxidized metal.
Some deoxidation methods also include treating the metal with slag in a ladle and vacuuming.
The effectiveness of using a particular deoxidation method (and, consequently, the total oxygen content) is determined by the deoxidizing ability of the deoxidizing element used and the degree to which the system approaches equilibrium. The latter, in turn, depends on the kinetics of deoxidation reactions, the rate of nucleation and removal of deoxidation products from the liquid metal, and on the interaction of the melt with coexisting phases.
You can submit comments, comments and questions about this article here. We will definitely answer you.
Classification of steels [edit | edit code ]
Steel that has been completely deoxidized is called calm, steel that has not been deoxidized at all is called boiling, steel that has not been completely deoxidized is called semi-quiet.
Steel is the main metal material used in the production of machines, tools and appliances. Its widespread use is explained by the presence in this material of a whole complex of valuable technological, mechanical and physicochemical properties. In addition, steel has a relatively low cost and can be produced in large quantities. The production process of this material is constantly being improved, thanks to which the properties and quality of steel can ensure trouble-free operation of modern machines and devices at high operating parameters.
General principles for classifying steel grades
The main classification characteristics of steels: chemical composition, purpose, quality, degree of deoxidation, structure.
- Steels according to their chemical composition into carbon and alloy. Based on the mass fraction of carbon, both the first and second groups of steels are divided into: low-carbon (less than 0.3% C), medium-carbon (C concentration is in the range of 0.3-07%), high-carbon - with a carbon concentration of more than 0.7%.
Alloyed steels are those that contain, in addition to permanent impurities, additives introduced to increase the mechanical properties of this material.
Chromium, manganese, nickel, silicon, molybdenum, tungsten, titanium, vanadium and many others are used as alloying additives, as well as a combination of these elements in various percentages. Based on the number of additives, steels are divided into low-alloy (alloying elements less than 5%), medium-alloy (5-10%), and high-alloy (contain more than 10% additives).
- According to their purpose, steels are classified as structural, instrumental and special-purpose materials with special properties.
The most extensive class are structural steels , which are intended for the manufacture of building structures, parts of devices and machines. In turn, structural steels are divided into spring-type, improved, cemented and high-strength.
Read also: Abstract on the topic of calipers
Tool steels are distinguished depending on the purpose of the tool produced from them: measuring, cutting, hot and cold deformation dies.
Special-purpose steels are divided into several groups: corrosion-resistant (or stainless), heat-resistant, heat-resistant, electrical.
- According to the quality of steel, there are ordinary quality, high-quality, high-quality and especially high-quality.
The quality of steel is understood as a combination of properties determined by the process of its manufacture. Such characteristics include: uniformity of structure, chemical composition, mechanical properties, manufacturability. The quality of steel depends on the content of gases in the material - oxygen, nitrogen, hydrogen, as well as harmful impurities - phosphorus and sulfur.
- According to the degree of deoxidation and the nature of the solidification process, steels are calm, semi-calm and boiling.
Deoxidation is the operation of removing oxygen from liquid steel, which provokes brittle fracture of the material during hot deformation. Mild steels are deoxidized with silicon, manganese and aluminum.
- According to their structure, steels are divided into annealed (equilibrium) and normalized states. Structural forms of steels are ferrite, pearlite, cementite, austenite, martensite, ledeburite and others.
Calm steel
In English, calm steel is called slightly “intimidating” - killed
steel
. Calm steel is steel in which there is virtually no gas emission when the ingot solidifies after casting. This is ensured by complete deoxidation of the steel - the complete removal of oxygen from it and the formation of a shrinkage cavity in the upper part of the ingot. This part of the ingot is then cut off and scrapped.
All alloy steels, most low alloy steels and many carbon steels are commonly used as mild steels. During continuous casting, the steel is also “calmed” completely. Calm steel is characterized by a homogeneous structure and uniform distribution of chemical composition and properties.
To obtain mild steel, it is deoxidized with aluminum, as well as manganese or silicon ferroalloys. In addition, calcium silicide and other special deoxidizers are sometimes used.
The influence of carbon and alloying elements on the properties of steel
Industrial steels are chemically complex alloys of iron and carbon. In addition to these basic elements, as well as alloying components in alloy steels, the material contains permanent and random impurities. The main characteristics of steel depend on the percentage of these components.
In our price list you can find out the current cost of fittings in St. Petersburg and the Leningrad region.
Carbon has a decisive influence on the properties of steel. After annealing, the structure of this material consists of ferrite and cementite, the content of which increases in proportion to the increase in carbon concentration. Ferrite is a low-strength and ductile structure, while cementite is hard and brittle. Therefore, an increase in carbon content leads to an increase in hardness and strength and a decrease in ductility and toughness. Carbon changes the technological characteristics of steel: workability by pressure and cutting, weldability. An increase in carbon concentration leads to deterioration in machinability due to hardening and reduced thermal conductivity. The separation of chips from high-strength steel increases the amount of heat generated, which causes a decrease in tool life. But low-carbon steels with low viscosity are also poorly processed, since chips that are difficult to remove are formed.
Steels with a carbon content of 0.3-0.4% have the best cutting machinability.
An increase in carbon concentration leads to a decrease in the ability of steel to deform in hot and cold states. For steel intended for complex cold forming, the amount of carbon is limited to 0.1%.
Low-carbon steels have good weldability. For welding medium- and high-carbon steels, heating, slow cooling and other technological operations are used to prevent the occurrence of cold and hot cracks.
To obtain high strength properties, the amount of alloying components must be rational. Excess alloying, excluding the introduction of nickel, leads to a decrease in toughness reserve and provokes brittle fracture.
- Chromium is a non-deficient alloying component and has a positive effect on the mechanical properties of steel at its content of up to 2%.
- Nickel is the most valuable and scarce alloying additive, introduced in a concentration of 1-5%. It most effectively reduces the cold brittleness threshold and helps to increase the temperature reserve of viscosity.
- Manganese, as a cheaper component, is often used as a substitute for nickel. Increases yield strength, but may make steel sensitive to overheating.
- Molybdenum and tungsten are expensive and scarce elements used to increase the heat resistance of high-speed steels.
Flotation of deoxidation products
The use of deoxidizers other than carbon leads to the formation of liquid or solid products in the form of a dispersed phase in the molten steel. Since these oxides are lighter than steel, they rise to the surface of the melt and can be removed as slag. Typically, particles with a radius of less than 10-3 cm are not able to rise to the surface of the melt; particles with a radius of more than 10-2 cm are almost completely removed from the melt. To effectively remove particles, measures are taken to coalesce them into larger particles.
Principles of steel marking according to the Russian system
In the modern metal products market there is no common steel marking system, which significantly complicates trading operations, leading to frequent errors when ordering.
In Russia, an alphanumeric designation system has been adopted, in which the names of the elements contained in steel are marked with letters, and their quantities are marked with numbers. The letters also indicate the method of deoxidation. The marking “KP” denotes boiling steels, “PS” – semi-calm steels, and “SP” – calm steels.
- Ordinary quality steels have an index St, after which a conditional grade number from 0 to 6 is indicated. Then the degree of deoxidation is indicated. The group number is placed in front: A – steel with guaranteed mechanical characteristics, B – chemical composition, C – both properties. As a rule, the group A index is not assigned. Example of designation – B Article 2 KP.
- To designate structural high-quality carbon steels, a two-digit number indicating the C content in hundredths of a percent is indicated in front. At the end - the degree of deoxidation. For example, steel 08KP. High-quality tool carbon steels have the letter U in front, and then a double-digit carbon concentration in tenths of a percent - for example, U8 steel. High-quality steels have the letter A at the end of the grade.
- In alloy steel grades, letters indicate alloying elements: “N” is nickel, “X” is chromium, “M” is molybdenum, “T” is titanium, “B” is tungsten, “Y” is aluminum. In structural alloy steels, the C content is indicated in hundredths of a percent at the front. In tool alloy steels, carbon is marked in tenths of a percent; if the content of this component exceeds 1.5%, its concentration is not indicated.
- High-speed tool steels are designated by the index P and an indication of the tungsten content in percent, for example, P18.
Read also: Electric oven power kW
Marking of steels according to American and European systems
Are you planning to buy rolled metal? Our store has reasonable prices and manufacturer's quality.
In the United States, there are several steel marking systems developed by various standards organizations. For stainless steels, the AISI system is most often used, which is also valid in Europe. According to AISI, steel is designated by three numbers, in some cases followed by one or more letters. The first number indicates the class of steel, if it is 2 or 3, then it is austenitic class, if 4 it is ferritic or martensitic. The next two digits indicate the serial number of the material in the group. The letters stand for:
- L – low mass fraction of carbon, less than 0.03%;
- S – normal concentration of C, less than 0.08%;
- N means nitrogen has been added;
- LN – low carbon content combined with nitrogen addition;
- F – increased concentration of phosphorus and sulfur;
- Se – steel contains selenium, B – silicon, Cu – copper.
In Europe, the EN system is used, which differs from the Russian one in that it first lists all alloying elements, and then, in the same order, their mass fraction is indicated in numbers. The first number is the carbon concentration in hundredths of a percent.
If alloy steels, structural and tool, except for high-speed steels, include more than 5% of at least one alloying additive, the letter “X” is placed in front of the carbon content.
EU countries use the EN marking, in some cases indicating in parallel the national mark, but with the mark “obsolete”.
Steel is a malleable and wrought alloy of iron and carbon (as a permanent impurity). Also contains other alloying elements and other harmful impurities. The carbon content should not exceed 2.14%. By changing the chemical composition of this alloy using carbon concentration and adding alloying elements, it is possible to obtain a wide range of different grades of this metal that will have different properties. This is what allows this material to be used in most industries.
Principles of steel classification
Classification and marking of steel occurs according to the following parameters:
- chemical composition;
- structure;
- appointment;
- quality and method of production;
- degree of deoxidation.
By chemical composition
Depending on the chemical composition, this metal is divided into two types: carbon and alloy. In turn, carbonaceous materials are divided into:
- low-carbon (carbon content below 0.2%);
- medium-carbon (carbon content in the range of 0.2% - 0.45%);
- high-carbon (carbon content above 0.5%).
Alloy steels are classified according to the total total amount of alloying elements (the carbon content is not summed up; manganese begins to be considered an alloying element when its content in the alloy is more than 1%, silicon - more than 0.8%). The following are distinguished:
- low alloy (below 2.5%);
- medium alloyed (within 2.5% - 10%);
- highly alloyed (more than 10%).
By structure
Such a classification feature as the structure of the material is considered less stable, since it depends on the cooling rate, alloying, heat treatment method and some other variable factors. However, the structure of the finished material still allows for an objective assessment of its quality. Classification of steel by structure in the annealing and normalization states. In the annealing state, the following are distinguished:
Read also: Which accuracy class is higher than 1 or 2
- hypoeutectoid - contains excess ferrite in its structure (for example, used for hot deformation dies);
- eutectoid - the structure consists of perite;
- hypereutectoid - contains secondary carbides in the structure (mostly used in the manufacture of tools);
- carbide (or ledeburite) - the structure contains primary carbides (for example, high-speed cutting);
- ferritic (stainless, heat-resistant and others);
- austenitic.
After the normalization process, steel is divided into the following classes:
- pearlitic - contain a low amount of alloying elements, structure after normalization: pearlite, pearlite + ferrite, pearlite + hypereutectoid carbide;
- martensitic - contain a high amount of alloying elements, as well as a relatively low critical hardening rate;
- austenitic - characterized by a high content of alloying elements, structure: austenite, austenite + carbide.
By purpose
Based on their purpose, steels are divided into structural, instrumental and special purpose (having special properties).
Structural ones are used for the manufacture of all kinds of parts in devices, machines, and elements of building structures. They are divided into:
- ordinary quality;
- improved;
- cemented;
- automatic;
- high strength;
- spring-spring.
Tools are used for the manufacture of cutting, measuring and other tools. They are divided into the following groups:
- for the manufacture of cutting tools;
- for the manufacture of measuring instruments;
- for the manufacture of stamping and pressing equipment.
Special purpose are alloys with special physical and/or mechanical properties. There are:
- stainless (corrosion-resistant);
- heat resistant;
- heat resistant;
- wear-resistant;
- magnetic;
- non-magnetic, etc.
By quality and production method
In this case, quality is understood as the entire set of properties of the metal, which are determined by the metallurgical process of its manufacture. The quality of steel is determined by the presence of harmful impurities in it. First of all, these are the chemical elements sulfur and phosphorus. Depending on their content they are divided into:
- ordinary quality - containing up to 0.06% sulfur and 0.07% phosphorus;
- high-quality - up to 0.035% sulfur and 0.035% phosphorus;
- high-quality - no more than 0.025% sulfur and 0.025% phosphorus.
- especially high quality - no more than 0.015% sulfur and 0.025% phosphorus.
According to the degree of deoxidation
Deoxidation is the process of removing oxygen from a liquid alloy. Undeoxidized steel has relatively low ductility and is more susceptible to brittle fracture during heat treatment under pressure. According to the degree of deoxidation they are divided into:
The process of deoxidizing still steels in a smelting furnace/or ladle using manganese, aluminum and silicon. Solidification in the mold occurs quietly, without gas evolution. A shrinkage cavity is formed in the upper part of the ingots. This type has anisotropy, that is, the mechanical properties are different and depend on the direction - the plastic properties in the transverse direction (along the rolling direction) are significantly lower than in the longitudinal direction. In addition, in the upper part of the ingot the content of sulfur, phosphorus and carbon is increased, and in the lower part it is reduced. This significantly worsens the properties of the product, sometimes even to the point of rejection.
Deoxidation in boiling water occurs only due to manganese. Excess oxygen during solidification partially reacts with carbon, releasing gas bubbles (carbon monoxide). This is where the impression of “boiling” is created. In this type there are practically no non-metallic inclusions arising from deoxidation products. It is a low-carbon alloy, with a minimum silicon content and a high content of gaseous impurities. Used in the manufacture of car body parts, etc. It has good cold formability.
Semi-quiet steels occupy a middle position between calm and boiling steels. Deoxidation is carried out in two stages: partly in the melting furnace and ladle, and finally in the mold. In the mold, deoxidation occurs due to the carbon contained in the metal.
Method for deoxidation of crack-sensitive steel
OP HCAHHE
INVENTIONS
FOR THE AUTHOR'S CERTIFICATE
Union of Soviets
Socialist
Republic poppy
F==-.: p. (61) Additional to auto. certificate (22) Declared 230281 (21) 3253194/22-02 (51) M. Kl.z with the addition of application ¹
S 21 S 5/52
State Committee
THE USSR. for inventions and discoveries (23) Priority
Published 09/07/82. Bulletin No. 33
Date of publication of the description 07.0982 (53) UDC 669. 187. .25(088.8) B.R. Komelkrv, G.B. Shearer, i3.A. Salauti
t0.Ô. Komov, V.V. Lasenko, Yu.V. Borisov, A.B. Gubin and B.S. Petrov (72) Inventors
Central Order of Labor Red Sign Research Institute of Ferrous Metal named after. I.P. Bardina (71) Applicant (54) METHOD FOR DECOKIDING CRACK SENSITIVE
STEEL with
The invention relates to ferrous metallurgy, namely to the smelting of crack-sensitive structural steel in an electric arc furnace, especially for subsequent electroslag remelting.
The smelting of crack sensing is known. solid structural steel grades
50G, 40ХН, 40Х, 45, 40 in 25 t electric arc furnaces for subsequent electroslag remelting with replacement of metal deoxidation with aluminum by deoxidation and microalloying of steel in a ladle with titanium. To do this, TiV grade ferrotitanium with a titanium content of at least 60b and an aluminum to titanium ratio of no more than 0.07 1 j is placed on the bottom of the ladle.
This method uses highly scarce high-percentage ferrotitanium, which does not allow expanding the production of crack-sensitive steels using this technology.
It is also known to add silicocalcium fraction of no more than 50 mm to slag during steel smelting in main arc furnaces (2).
The addition of lump silicocalcium to the slag in an operating arc furnace leads to the loss of calcium in the area of influence of electric arcs on the slag, since calcium is an element with high vapor elasticity at steelmaking temperatures.
As a result, the beneficial use of calcium as a deoxidizer is reduced.
The closest to the proposed technical essence and achieved result is the method of deoxidation of steel smelted in an electric arc furnace for subsequent electroslag remelting, which includes preliminary deoxidation of the metal with lump ferrosilicon, deoxidation of slag with coke and high-percentage ferrosilicon powders and final deoxidation of steel with silico-calcium and aluminum in release process (3).
Aluminum is introduced mounted on iron rods 0.7 kg/t, silicocalcium with a particle size of no more than 50 mm 1.0 kg/t.
Silicocalcium is added under a stream of metal.
The disadvantages of this method of steel deoxidation are the need for special breaking of silicocalcium into powder, which is associated with the danger of explosion, especially when grinding silicocalcium ma956575 ro "SK20-30 with a calcium content of more than 20b in a ball mill, as well as the use of silicocalcium with a particle size of no more than 50 mm, i.e. containing also a fine fraction, which is partially carried out of the ladle by heat flows. As a result, the beneficial use of calcium is reduced. Deoxidation of steel with aluminum in an amount of 0.7 kg/t causes the formation of aluminum nitrides in the metal, which are the main cause of cracks in electroslag
I ingots obtained from the original steel, deoxidized with a significant amount of aluminum. In addition, a disadvantage of the steel deoxidation method is the small amount of silicocalcium used for the final deoxidation of the metal in the ladle, since such an amount of silicocalcium does not prevent the formation of aluminum nitrides in the steel due to the interaction of calcium with metal nitrogen.
The purpose of the invention is to increase the efficiency of calcium use and reduce the rejects of electroslag ingots along longitudinal cracks.
This goal is achieved by the fact that according to the method of deoxidation of crack-sensitive steel, smelted in an electric arc furnace for subsequent electroslag remelting, including preliminary deoxidation of the metal with lump ferrosilicon, deoxidation of slag with coke powders and 35 high-percentage ferrosilicon and final deoxidation of steel with silicocalcium and aluminum: during the production process, before After the melt is released, silicocalcium with a particle size of up to 20 mm is added to the refining slag in a switched off furnace in an amount of 10-20b of its total consumption, equal to 1.5-3.0 kg/t, the remaining amount of silicocalcium in pieces with a particle size of 20-100 mm is introduced 45 with a feed rate of 2-4 kg/t.min into the steel-pouring ladle onto the metal stream while draining the first third of the melt and at the same time deoxidizes the steel with aluminum in the amount of 0.10.3 kg/t„, while the aluminum is placed evenly along the height of the ladle at a distance from its bottom within
1/8-1/3 of its height.
The addition of a silicocalcium fraction of less than 20 mm to the refining slag in a switched-off furnace ensures high deoxidation of the outlet refining slag. Due to this, during the introduction of silicocalcium with a particle size of 20-100 mm, it eliminates the removal of 60 small fractions of silicocalcium from the ladle, which increases the beneficial use of calcium.
Simultaneous deoxidation of steel in a ladle with silicocalcium and aluminum 65 helps reduce calcium loss.
As a result, favorable conditions are created for the interaction of calcium with metal nitrogen. This reduces the likelihood of aluminum nitrides forming in steel, which is one of the main causes of longitudinal cracks in electroslag ingots.
Deoxidation of steel with aluminum before deoxidation with silicocalcium increases the likelihood of the formation of unfavorable nitrides. Deoxidation of steel with aluminum after deoxidation with silicocalcium reduces the beneficial use of calcium. Treating steel with calcium increases its ductility.
This has a positive effect on the metal’s resistance to cracking.
The introduction of silicocalcium and aluminum into the ladle during the draining of the first third of the melt promotes better mixing of elements - deoxidizers - with the metal and averaging their content in the steel.
Silicocalcium consumption is less
1.5 kg/t does not provide a sufficient reduction in the rejects of electroslag ingots along longitudinal cracks. The use of silicocalcium in an amount of more than 3 kg/t is irrational, since when silicocalcium contains 70-75B silicon, treating metal with silicocalcium in a ladle increases its silicon content by more than 0.150.20B, and this limits the use of high-percentage ferrosilicon powder for deoxidation of slag in an electric arc ovens.
An additive before releasing the melt onto the refining slag into the silico-calcium shut-off furnace in an amount of less than
10b of its total consumption does not ensure the deoxidation of refined slag to a ferrous oxide content of less than 0.5b. Adding silicocalcium to refined slag in a switched-off furnace in an amount of more than 20b of its total consumption is impractical, since it reduces the amount of silicocalcium consumed for processing steel in the ladle.
The introduction of silicocalcium with a particle size of less than 20 mm into a steel-pouring ladle leads to the removal of fine fractions and reduces the useful use of calcium. The introduction of silico-calcium with a particle size of more than 100 mm into a steel-pouring ladle is not rational, since it increases the time for melting of silico-calcium, thereby reducing the time of interaction of calcium with steel.
The lower limit of the intensity of silicocalcium introduction into a steel-pouring ladle (2 kg/t.min) is determined by the need to add the entire amount of silicocalcium in a time not exceeding 956575
25 The duration of draining a third of the melt. The introduction of silicocalcium into the ladle at a rate of more than 4 kg/t.min leads to a decrease in the temperature of the slag-metal melt and is associated with the danger of the formation of a slagged conglomerate from pieces of silicocalcium.
Deoxidation of steel in a ladle with aluminum in an amount of less than 0.1 kg/t does not provide the required austenite grain of 6-8 points in the steel, and deoxidation with aluminum in an amount of more than 0.3 kg/t is uneconomical and increases the likelihood of the formation of unfavorable aluminum nitrides . 15
Placing aluminum at a distance from the bottom of the ladle of less than 1/8 of its height is impractical, since the first portions of metal and slag are cooled in the ladle more than the subsequent ones, and aluminum dissolves in them slowly, 1 which is associated with this slagging. Distribution of aluminum at a distance from the bottom of the bucket of more than 1/3
its height does not ensure effective mixing and uniform distribution of aluminum in steel.
The relevance of using the invention in the smelting of crack-sensitive structural steel grades
12-2ОХНЭА, ЗОХГСА, 50Г, 40ХН, 45, 40, etc. in electric arc furnaces with a capacity of 25-200 tons is determined by the possibility, as experimental melts show, to significantly reduce and even completely eliminate the rejection of electroslag ingots of these steels due to the defect longitudinal crack. This eliminates the need to use scarce ferro-40 titanium grade TiV in the smelting of crack-sensitive steels with an actual titanium content of 70% and an aluminum-to-titanium ratio of no more than 0.07.
Example 1. When smelting in
25 tons of arc furnace steel 20ХНЗА.metal 45 at the beginning of the refined cycle are preliminarily deoxidized with lump 45% ferrosilicon and calculation of the introduction
0.15% silicon. Refining slag, induced with lime 25 kg/t, fireclay and fluorspar 5 kg/t, is deoxidized with coke powders 1-3 kg/t and 65b ferrosilicon 3-5 kg/t. Before releasing the melt into the switched-off furnace, silico-calcium grade SK30 with a particle size of up to 20 mm is added to the refining slag in an amount of 10% of
its total consumption is 3 kg/t, i.e.
0.3 kg/t or 7.5 kg per melt and mix the slag with a wooden paddle. During the release of the first third of the melt, the remaining silico-calcium grains of 20-100 mm in quantity are introduced into the steel ladle onto the metal stream
2.7 kg/t, i.e. 67.5 kg per melt, with a feed rate of 4 kg/t, min, – e..synthesis is introduced in 40 s.
Duration of the entire episode
2.5 min. Simultaneously with the introduction of silicocalcium, the steel in the ladle is deoxidized with aluminum in the amount of 0.3 kg/t, i.e. 7.5 kg per melt, for which pig aluminum in pieces is no more
4 kg, mounted on a rod, is fixed on the side of the bucket so that the pieces of aluminum are located evenly in height at a distance from the bottom of the bucket ranging from 1/8 to 1/4 of the height of the bucket. Steel is poured into consumable electrodes with a cross-section of -370 x 370 mm in a semi-continuous steel casting plant. After electroslag remelting of electrodes, the defective longitudinal crack of ingots with a cross-section of 550 x 550 mm and a weight of 5.5 tons of 20KhNZASH steel is reduced from 20 to 2%.
Example 2. When smelting in
100 tons of arc furnace steel ZOXGSA metal in the beginning of the refining period is preliminarily deoxidized with lump 45% ferrosilicon at the rate of introduction of 0.20b silicon. Lime-induced refining slag
25 kg/t, fireclay and fluorspar 5 kg/t, deoxidized with coke powders 1-3 kg/t and 65b ferromilitium 3-5 kg/t. Before releasing the melt, silicocalcium with a particle size of up to 20 mm is added to the refining slag in a switched off furnace in an amount of 15% of its total consumption, equal to
2.5 kg/t, i.e. 0.37 kg/t or 37 kg per melt, and mix the slag with a wooden paddle. At the time of release of the first third of the melt, the remaining silico-calcium coarseness is introduced into the steel-smelting ladle onto the metal stream
20-100 mm in an amount of 2.13 kg/t or 213 kg per heat, with a feed rate of 2.5 kg/t min. those. silicocalcium is administered over 52 s.
Duration of the entire episode
4 min. Simultaneously with the introduction of calcium silicone, the steel in the ladle is deoxidized with aluminum in the amount of 6.15 kg/t, i.e.
15 kg per melt, for which pig aluminum in pieces of no more than 4 kg, mounted on rods, is fixed on the side of the ladle so that the pieces of aluminum are located evenly in height at a distance from the bottom of the ladle within 1/6-1/3 of the height of the ladle. Steel is poured by siphon into ingots weighing
6.5 tons and rolled onto consumable electrodes with a cross-section of 370 x 370 mm. After electroslag remelting of the electrodes, the defective ingots with a cross-section of 550 x x 550 mm, weighing 5.5 tons of ZOKHGSASH steel according to the defect, the longitudinal crack is reduced from 10 to 1%.
Example 3. When smelting in
200 t arc furnace. steel 40Х metal at the beginning of the refining period before 956575
Compiled by L. Iagayumova
Editor N. Kishtulinets Techred AA÷ Proofreader G. Reshetnik, o
°
Order 6522/6 Circulation 587 Subscription
VNIIPI of the USSR State Committee for Inventions and Discoveries
113035, Moscow, Zh-35, Raushskaya embankment, 4/5
Fi.pial PPP "Patent", Uzhgorod, st. Proyektnaya, 4 boiling deoxidize in lumps
45% ferrosilicon based on the introduction of 0.15% silicon. Refining slag, made from lime 22.5 kg/t, fluorspar or fireclay up to 10 kg/t each, is deoxidized with coke powders 3 kg/t and 653 ferrosilicon 4 kg/t. Before releasing the melt, silico-calcium, with a particle size of up to 20 mm, is added to the refining slag and the switched-off furnace in an amount of 20% of 10 of its total consumption of 1.5 kg/t, i.e.
0.3 kg/t or 60 kg per melt, and mix the slag with a wooden paddle. During the release of the first third of the melt, the remaining silico-calcium is introduced into the steel-pouring ladle with 15 streams of metal. tions with a particle size of 20-100 mm in quantity
1.2 kg/t, i.e. 240 kg per melt, with a feed rate of 2 kg/t min, i.e. silicocalcium is administered over 72 s. The duration of the entire release is 7 minutes. Simultaneously with the introduction of silicocalcium, the steel is deoxidized in the ladle with aluminum in the amount of 0.1 kg/t, i.e. 20 kg per melt, for which pig aluminum in pieces of no more than 8″ kg, mounted on rods, is fixed on the side of the ladle so that the pieces of aluminum are located evenly along the hole at a distance from the bottom of the ladle within 1/6 1/4 of the height of the Zo bucket. Steel is siphoned into ingots weighing 6.5 tons and rolled onto consumable electrodes with a cross section of 370 x
370 mm. After electroslag transfer melt electrodes scrap ingots with a cross section of 550 x 550 mm weighing 5.5 tons of steel
40ХШ along the defect, the longitudinal crack is reduced from 7 to 0%.
The economic efficiency of the proposed method is determined by the reduction of losses due to the reduction of rejects of 10 electroslag ingots of crack-sensitive structural steels for the defect longitudinal crack and amounts to 420 thousand rubles/r. With a volume of electroslag remelting of crack-45 sensitive steels of 20 thousand t/y, the weighted average cost of electroslag ingots of these steels
280 rub./t and a weighted average reduction in the rejects of electroslag ingots for these steels from 9 to 1.5%, the economic efficiency of the proposed invention for a year at one site of metallurgical plants of high-quality metallurgy will be:
20.000 x 280 x 0.09 – 20.00 x 280 x . x 0.015 = 420 thousand rubles.
Claim
A method of deoxidation of crack-sensitive gel steel smelted in an electric arc furnace for subsequent electroslag remelting, including preliminary deoxidation of the metal with lump ferrosilicon, deoxidation of slag with coke powders and high-percentage ferrosilicon and final deoxidation of steel with silicocalcium and aluminum during the production process, which usually results that, in order to increase the efficiency of using calcium and reduce the rejects of electroslag ingots along longitudinal cracks, before releasing the melt, silico-calcium with a particle size of up to 20 mm is added to the refining slag in an amount of
10-20% of its total consumption, equal to 1.5-3.0 kg/t, the rest of the amount of silicocalcium in pieces of fine size
20-100 mm is introduced with a feed intensity of 2-4 kg/t min into a steel-pouring ladle onto the metal stream during the draining of the first third of the melt and at the same time deoxidizes the steel with aluminum in an amount of 0.1-0.3 kg/t, while the aluminum placed evenly along the height of the bucket at a distance from the bottom of the bucket within 1/8-1/3 of its height.
Sources of information taken into account during the examination
1. Improvement of technological modes for smelting alloy steels in 25-ton high-power arc furnaces, semi-continuous casting of consumable electrodes and their electroslag remelting. TsNIICHI report. M., 1980, R state registration
79008562, archived. R 7035.
2. General technological instructions for steel smelting in basic arc furnaces, R S-0-77, ch. 17, g 17, 12.
3. Instructions for the smelting of structural steels for critical purposes R S-9-77.
Decoding steels in materials science
Belongs to the class: structural carbon quality. Chemical composition: carbon - 0.17−0.24%; silicon - 0.17−0.37%; manganese - 0.35−0.65%; sulfur - up to 0.04%; phosphorus - up to 0.04%. Widely used in boiler making, for pipes and heating pipelines for various purposes; in addition, the industry produces rods and sheets.
HVG transcript
Belongs to the class: alloyed instrumental. Used for the manufacture of measuring and cutting tools, taps, broaches.