Chemical passivation as an optimal coating for heat-resistant steel
Metal passivation is a process as a result of which an oxide film is formed on the surface of the metal, preventing the formation of corrosion.
The name of the coating method comes from the word “passivity”. The purpose of passivation is to reduce the chemical reactivity of a metal when interacting with other metals or aggressive environmental conditions. In its own way, the appearance of a film is the same destruction of metal. But by destroying the top layer of material by several tens of nanometers, passivation saves the lower layers from rust.
Thus, chemical passivation is the interaction of an oxidizing agent with the surface being treated.
Stages of chemical passivation
1. If you do not prepare the metal product first, the oxidizing agent will react not with the alloy, but with foreign elements. Therefore, before passivation it is necessary to clean the surface . Cleaning is carried out in 2 ways: by washing or sanding the product using sandpaper. Now you can start passivation.
2. The process itself involves applying a chemical reagent to the product . A protective film is formed on the alloy, consisting mainly of salts and oxides. The film makes the structure of the product stronger and more durable. The effectiveness of the procedure depends on the following factors:
- composition of the solution;
- alloy composition;
- condition of the surface of the workpiece.
High-alloy steels, especially chromium-nickel steels, are best suited to chemical passivation. But carbon steels should be treated only for short-term protection, since the level of the protective layer on them is significantly weaker.
3. Cleaning with water . Any salts that may remain on the product may cause corrosion. Therefore, washing should be carried out carefully.
4. Residual acid must be neutralized with a 2-3% ammonia solution or a solution consisting of 25-30 g/l oleic acid and 2-4 g/l sodium hydroxide. Treatment is carried out at 80 – 90 °C for 2-3 minutes.
What solution is used?
The use of different solutions depends on the properties of the alloy. Let's consider what solutions are used to passivate various classes of ferrous metals:
Highly alloyed alloys resistant to corrosion - nitric and sulfuric acids.
- Ferritic alloys - potassium dichromate, nitric acid.
- Carbon steels - potassium dichromate, chromic anhydride, phosphoric acid, sodium hydroxide.
- Medium alloy steels - chromic anhydride, phosphoric acid.
The passivation temperature and time also depend on the alloy class. The temperature ranges from 18 to 90 °C, and the time ranges from 3 to 60 minutes.
The higher the temperature of the solution, the faster the process proceeds.
Application of passivation
- Passivation is used for metal parts for painting. It not only protects against corrosion, but also degreases products. Used in the field of mechanical engineering.
- Passivation of steam turbines. But why do you need passivation of stainless steel, since it won’t rust anyway? It turns out that if the alloy is in continuous contact with an aggressive environment, it can collapse. An example is a weld. Sometimes there are iron particles on it. And then even stainless steel corrodes.
- Dental field. The lower part of the implants is processed - the screws that are mounted into the jaw. Passivation is used to prevent destruction of the implant in the jawbone.
- Chemical passivation is often carried out for decorative purposes. With short-term treatment, a rainbow film appears on the surface. Bright objects of use - taps, door handles.
- Passivation of costume jewelry is used to avoid allergic reactions.
Chemical passivation significantly extends the service life of metal products and deserves wide application in a wide variety of fields.
How to pass stainless steel
This can make the difference between satisfactory performance and premature failure. If done incorrectly, passivation can actually cause corrosion. Passivation is a post-fabrication technique that maximizes the inherent corrosion resistance of the stainless alloy from which the workpiece is made. This is not a scale removal procedure and it does not look like a coat of paint. There is no universal agreement regarding the exact mechanics of how passivation works. But there is no doubt that there is a protective oxide film on the surface of passive stainless steel. This invisible film is said to be extremely thin, less than 0.0000001 inches thick, which is about 1/100,000th the thickness of a human hair! A clean, newly machined, polished or pickled stainless steel part will automatically acquire this oxide film from exposure to oxygen in the atmosphere.
Under ideal conditions, this protective oxide film completely covers all surfaces of the part. However, in practice, contaminants such as dirt or iron particles from cutting tools may be transferred to the surface of stainless steel parts during processing. If not removed, these foreign particles may reduce the effectiveness of the original protective film. During the machining process, microscopic amounts of free iron can be rubbed off the cutting tool and transferred to the surface of the stainless steel workpiece. Under certain conditions, a thin coating of rust may appear on the part. This is actually corrosion of the steel from the tool rather than the base metal. Sometimes a gap in an embedded piece of steel from a cutting tool or its corrosion products can cause an attack on the part itself. Likewise, small particles of iron-containing solid dirt can adhere to the surface of the part. Although the metal may appear shiny when machined, invisible particles of free iron can cause the surface to rust after exposure to air. Open sulfides can also be a problem. They come from the addition of sulfur to stainless steels to improve machinability. Sulfides improve the alloy's ability to form chips that are completely removed from the cutting tool during the machining process. If the part is not properly passivated, sulfides can act as corrosion initiation sites on the surface of the fabricated product. In both cases, passivation is required to maximize the natural corrosion resistance of the stainless steel. It can remove surface contaminants such as ferrous solid dirt and iron particles from cutting tools that may form rust or act as initiation sites for corrosion. Passivation can also remove sulfides exposed on the surface of stainless alloys without mechanical treatment. A two-step procedure can provide the best corrosion resistance: 1. cleaning, a fundamental but sometimes overlooked procedure, and 2. acid bath or passivation treatment.
Cleaning The first cleaning should always be the first. Grease, coolant or other workshop debris must be thoroughly cleaned from the surface to obtain the best corrosion resistance. Processing chips or other magazine dirt can be thoroughly wiped off the part. A commercial degreaser or detergent can be used to clean used oils or coolants. Foreign matter such as thermal oxides may need to be removed by grinding or by methods such as acid pickling. Sometimes a machine operator may skip basic cleaning, mistakenly assuming that simply immersing an oiled part in an acid bath will accomplish cleaning and passivation at the same time. This doesn't happen.
Instead, the grease contaminant reacts with the acid to form gas bubbles. These bubbles collect on the surface of the workpiece and interfere with passivation. Worse, contamination of the passivation solution, sometimes with high chloride content, can cause a "flash" as shown in Figure 1. Instead of producing the desired oxide film with a shiny, clean, corrosion-resistant surface, the flash causes a heavily etched or darkened surface - a deterioration of the surface itself. surface, which is designed to optimize passivation. Parts made from martensitic stainless steels [which are magnetic, moderately resistant to corrosion, and capable of yield strengths up to 280 ksi (1930 MPa)] are hardened at high temperature and then quenched to provide the required hardness and mechanical properties . Precipitation hardenable alloys (which provide a better combination of strength and corrosion resistance than martensitic grades) can be solution processed, partially machined, held at lower temperatures, and then finished with machining. In such cases, the parts must be thoroughly cleaned with a degreaser or cleaner to remove traces of cutting fluid before heat treatment. Otherwise, the cutting fluid remaining on the parts will cause excessive oxidation. This condition can cause pitted bottoms to end up after descaling by acid or abrasive methods. If cutting fluids are allowed to remain on parts that are well cured, as in a vacuum oven or protective atmosphere, carburization of the surface may occur, resulting in loss of corrosion resistance. Passivation Baths After thorough cleaning, the stainless steel part is ready to be immersed in a passivation acid bath. Any of three approaches can be used: nitric acid passivation, nitric acid with sodium dichromate passivation, and citric acid passivation. Which approach to use depends on the grade of stainless steel and the prescribed acceptance criteria.
More stable chromium-nickel grades can be passivated in a bath of nitric acid (20% by volume) (Fig. 2). As indicated in the same table, less resistant stainless grades can be passivated by adding sodium dichromate to a nitric acid bath to make the solution more oxidizing and capable of forming a passive film on the surface. Another option, used instead of nitric acid plus sodium dichromate, is to increase the concentration of nitric acid to 50% by volume. The addition of sodium dichromate and a higher concentration of nitric acid reduce the likelihood of unwanted flare-ups.
The procedure for passivating non-machined stainless steels (also shown in Figure 2) is slightly different from the procedure used for non-machined stainless steels. This is because the sulfides of free-process sulfur-containing grades are partially or completely removed during passivation in a conventional nitric acid bath, creating microscopic breaks in the surface of the workpiece. Fig.2. The procedures for passivating stainless steel parts in nitric acid baths are quite simple. Nitric acid passivation of stainless steels - chromium-nickel alloy (300 series) - garnets with 17% chromium or more (except 440 series) 20% vol. nitric acid at 120/140°F (49/60°C) for 30 minutes. Illiterate chrome grades (12-14% chromium).
High carbon chrome granules (440 series). Precipitation Hardening Stainless steel 20% vol. nitric acid + 3 oz. per gallon (22 g/L) sodium dichromate at 120/40 °F (49/60 °C) for 30 minutes or 50 vol.%. nitric acid at 120/40°F (49/60°C) for 30 min. Passivation of stainless steel with free processing, including AISI types 420F, 430F, 440F, 203, 182-FM and Carpenter Project 70 + ® Types 303 and 416 1.5% by weight. sodium hydroxide at 160/180°F (71/82°C) for 30 minutes. 2. Washing water. 3.20% vol. nitric acid + 3 oz. per gal. (22 g/L) sodium dichromate at 120/40°F (49/60°C) for 30 minutes. 4. Washing water. 5.5% wt. sodium hydroxide at 160/180°F (71/82°C) for 30 minutes. 6. Washing water.
Even normally effective water washes can leave residual acid trapped in these gaps after passivation. This acid can then attack the surface of the part if it is not neutralized or removed. High Quality Grinding Wheels for Efficient Finishing To effectively passivate stainless steels without machining, Carpenter has developed an AAA (alkali-acid-alkaline) process that neutralizes trapped acid. This passivation method can be completed in less than 2 hours. Here's the step-by-step procedure: After degreasing, rinse the parts for 30 minutes in a 5 percent sodium hydroxide solution at 160 to 180°F (71°C to 82°C). Then rinse the part thoroughly with water. Then immerse the part for 30 minutes in a 20 percent nitric acid solution containing 3 ounces per gallon (22 g/L) sodium dichromate at 120 to 140 °F (49 °C to 60 °C). After removing the part from this bath, rinse it with water, then immerse it in the sodium hydroxide solution for another 30 minutes. Rinse the part with water and dry it using the AAA method. The benefits of this method are shown in Figure 3. Citric acid passivation is becoming increasingly popular among manufacturers who want to avoid the use of mineral acids or solutions containing sodium dichromate, along with the disposal problems and greater hazards associated with their use.
Citric acid is considered environmentally friendly in all respects. Although citric acid passivation offers attractive environmental benefits, shops that have had success with mineral acid passivation and have not experienced any safety concerns may want to stay aware. There may be no real need to change if these users have a clean shop, well-maintained and clean equipment, coolant free of ferrous magazine dirt, and a process that produces good results. Citric acid bath passivation treatment has been found to be applicable to a large number of stainless steel families, including several individual grades of stainless steel, as shown in Figure 4.
Conventional methods for passivation of nitric acid according to FIG. 2 included for convenience. Note that older nitric acid preparations are in percent by volume, while newer citric acid concentrations are in percent by weight. When performing these procedures, it is important to note that
What is passivation?
The passivation process allows stainless steel to return to its original properties, further protecting it from the effects of many external factors. This is a special chemical treatment of metal products, after which a special protective coating is formed on their surface. When interacting with concentrated acids, an inconspicuous film appears on stainless steel. This process is called passivation.
This method is used both for additional processing during the production of products and for restoring the basic properties of stainless steel parts.
Passivation of stainless steel
Passivation is a process separate from etching and can be done on its own or after etching. Unlike etching, the passivation process does not remove the surface layer of metal. Instead, the stainless steel surface is treated with an oxidizing acid to dissolve carbon steel, sulfide inclusions, and remove iron and other surface contaminants from the stainless steel. Passivation also promotes the formation of a high-chromium passive film, which provides corrosion resistance to stainless steel. While pickled steel will appear dull, properly done passivation will not affect or change the appearance of the metal.
Why is this necessary?
A stainless steel sheet has a very thin oxide film on its surface. It is this that prevents the formation of rust on parts, fasteners, and hardware made from this material. But the slightest violation of the integrity of this coating leads to the fact that the main anti-corrosion properties of stainless steel are lost. The causes of damage to the oxide film can be very different:
when the material comes into contact with chlorine; when steel interacts with sea water; in case of mechanical or physical damage, including scratches and minor dents.
Therefore, it is important to comply with the operating conditions that are regulated by the manufacturing plants of certain products (cutlery, fasteners, hardware, working tools, solid sheets, etc.). It is prohibited to use detergents containing chlorine and other aggressive chemicals.
But the greatest damage to the oxide film is caused by welding. This is especially harmful in the case of pipe welding. In such a situation, the protective surface is destroyed along the entire seam. Steel passivation is used to restore surfaces and protect products from rust. But here the composition of the stainless steel plays an equally important role.
First cleaning
- Grease, coolant or other contaminants must be thoroughly removed from the surface to obtain the best corrosion resistance. A commercial degreaser or detergent can be used to clean mechanical oils or coolants. Foreign matter such as thermal oxides may need to be removed by grinding or by methods such as acid etching.
- Sometimes an operator may skip basic cleaning, incorrectly assuming that simply immersing the grease in an acid bath will both clean and passivate simultaneously. This does not happen. Instead, the grease contaminant reacts with the acid to form gas bubbles. These bubbles collect on the surface of the workpiece and interfere with passivation.
- Even worse, contamination of the passivation solution, sometimes with high chloride content, can cause a corrosion “flare-up”. Instead of producing the desired oxide film with a shiny, clean, corrosion-resistant surface, flash causes a heavily etched or darkened surface—the deterioration of the surface itself that passivation is designed to optimize.
- Parts made from martensitic stainless steels [which are magnetic, moderately corrosion resistant and with a yield strength of up to (1930 MPa)] per square inch are hardened at high temperature and then annealed to provide the required hardness and mechanical properties. Precipitation hardenable alloys (which provide a better combination of strength and corrosion resistance than martensitic grades) can be solution processed, partially processed, held at lower temperatures, and then finished with machining.
- In such cases, the parts must be thoroughly cleaned with a degreaser or cleaner to remove traces of cutting fluid before heat treatment. Otherwise, cutting fluid remaining on the parts will cause excessive oxidation. This condition can cause the underlying layers to remain mottled even after descaling by acid or abrasive methods. Cutting fluids may remain on the parts and harden in a vacuum oven or protective atmosphere, and carburization of the surface may occur, resulting in loss of corrosion resistance.
- Etching
- Pickling is the removal of the adjacent low chromium metal layer from the surface of stainless steel by chemical means.
- Where steel is heated by welding, heat treatment or other means to the point that a colored oxide layer can be seen, there is a chromium-depleted layer on the surface of the steel below the oxide layer. Lower chromium content gives lower corrosion resistance. To restore the best corrosion resistance, the damaged metal layer must be removed, exposing the fully alloyed stainless steel surface. Mechanical removal may produce abrasive or other particles (corrosion inhibitors) or may be impractical, so chemicals are usually used.
- Procedures involving etching solutions of nitric (HNO3) and hydrofluoric (HF) acids remove the scale and chromium-depleted underlayer and restore corrosion resistance. Etching solutions also remove contaminants such as iron and iron particles. Etching solutions other than mixtures of nitric and hydrofluoric acids exist and can be used for specialized applications.
- Etching pastes, where a solution is mixed with an inert carrier, are typically used to treat selected areas such as welds.
- Etching involves the removal of metal and a change in the visual brightness of the metal.
- Electropolishing is a useful alternative to etching. Metal removal is achieved but usually results in a bright, smooth and more corrosion resistant surface.
Stainless steel classification
The anti-corrosion properties of stainless steel directly depend on its composition. Based on this, this steel is marked. The classification allows you to distinguish each type of stainless metal by flexibility, hardness, and degree of anti-corrosion protection. Depending on the composition and purpose, they are distinguished:
martensitic steels. Knives (including those for the food industry) and turbines are usually made from them. This steel, having a large percentage of chromium in its content, is very hard; ferritic materials. The amount of chromium in such steel exceeds the previous value by 3-4%. This material has high resistance to phosphoric acid, ammonium nitrate and nitric acid; austenitic steels. This type of stainless steel is very ductile. It is often used in mechanical engineering; duplex or ferro-austenitic metals. These are very durable, but at the same time flexible stainless materials.
What causes the high corrosion resistance of stainless steels?
The essence of such a phenomenon as corrosion is that the surface of the metal begins to deteriorate under the influence of negative external factors and the environment. Typically, corrosion due to constant oxidation affects the metal layer by layer, gradually destroying the internal structure of the steel. In many cases, it no longer makes sense to localize the affected areas of the internal structure of the metal, so steel products have to be replaced with new ones.
Passivation (or passivation), as a technology that allows for reliable protection of steel from corrosion, underlies the creation of such a unique metal as stainless steel. The chemical composition of the vast majority of steels belonging to the stainless category may contain various elements:
- nickel;
- molybdenum;
- cobalt;
- niobium;
- manganese.
However, the main alloying element of such steels, the amount of which in their composition can vary between 12–20%, is chromium. The addition of various alloying elements to the composition of stainless steels makes it possible to give them the required physical and chemical characteristics, but it is chromium that is responsible for the corrosion resistance of the steel alloy.
The effect of chromium on the properties of stainless steel
Stainless steel alloys, which contain 12% chromium, exhibit high corrosion resistance only when interacting with ambient air. If the amount of chromium in the chemical composition of stainless steel is increased to 17%, then products made from it can easily interact with nitric acid without losing their performance characteristics.
To make the metal resistant to even more aggressive environments, which include hydrochloric, sulfuric and other acids, the quantitative content of chromium in it is not only increased, but also elements such as copper, molybdenum, nickel, etc. are added to its composition. In other words , they passivate the metal, that is, increase its passivity to corrosion processes.
During the process of passivation of the weld zone, a strong film is formed
Passivation, in which appropriate alloying elements are added to the chemical composition of stainless steel, is not the only condition for high corrosion resistance of the metal. In order for the protective properties of stainless steel to remain at a high level, the oxide film on its surface, consisting mainly of chromium oxide, must be intact, have a uniform chemical composition and thickness.
Technology and methods
There are various methods for processing stainless steel. But there are two main methods of steel passivation:
Etching with chemical acids (concentrates) in certain areas. This technology is often used for processing welds, but is also allowed in other cases. This process has different processing sequence options. They differ both in the composition of chemicals and in the time of work. The most common method in this case is electrolytic etching. This technology consists of placing a stainless steel product in a specially prepared bath consisting of concentrated acids. An electric current (alternating or direct) is passed through this composition. The metal plays the role of either a cathode or anode. The supplied current has a mechanical effect on the steel, resulting in the release of hydrogen or oxygen gas. This helps to separate the oxide film on the surface of the product. Etching with ready-made acid mixtures. They can be made in the form of pastes, gels, sprays, concentrates. This method is the most convenient.
Regardless of which method is used to passivate stainless steel, it is important to follow the sequence of work.
When passivation is inappropriate
If a stainless steel product is not used in aggressive environments, then the use of acids and electric current retains the risk of deteriorating the quality of the metal surface for no apparent reason. Weigh carefully the economic feasibility of the passivation procedure and the real goals that need to be achieved.
Chemical passivation of stainless steel is possible only if you are completely sure of control over the process. In the case of a welded pipe, passivation of only the top layer of the seam does not guarantee sufficient protection on the inside of the seam. In this case, exposure to acids will accelerate and aggravate the process of rust formation.
Stages of chemical passivation
In the process of forming a homogeneous inert film on the surface of stainless steel products, it is important to take into account the characteristics of the steel composition and the degree of damage to the protective coating. Chemical passivation today is an integral part of working with stainless materials. This allows you to extend their service life, get rid of rust and damage, and prevent the formation of corrosion. During passivation work, the following sequence of steps should be followed:
First, the materials are cleaned from contaminants. Grease stains, rust and other deposits are removed. With chemical acid etching technology, the product is immersed in a bath with a mixture of hydrochloric acid and sulfuric acid. At temperatures from 60 to 80 degrees, the steel is kept here for 20-40 minutes. If the method of etching with ready-made mixtures of acids is used, then special concentrated compositions (pastes, gels, sprays) are used for cleaning, which are applied to the surface of the steel manually. The chemical is left for approximately 30 minutes. Then the products are thoroughly washed with water. The passivation process begins. In the first case, the steel is immersed in an acid bath. In the second, gels, pastes, sprays and other ready-made chemical compositions are applied to the surface of the product. In the case of ready-made products, one more stage is provided - treatment with a passivator. This allows for the forced formation of an oxide film on stainless steel. The last stage consists of thoroughly washing the product.
The composition of stainless steel and the grade play an important role in the appearance of the product after chemical passivation. Some species are dark in color, while others are lighter. But regardless of this, this method of steel processing has a whole list of advantages:
improves resistance to corrosion; the surface of the product is uniformly smoothed; burrs, scratches, dents are removed; The service life of the products is significantly increased.
What is stainless steel pickling?
Stainless steel pickling is the cleaning of a metal surface, which results in the actual removal of part of the surface layer of the metal in order to remove foreign inclusions, such as:
discoloration (welding oxides), free iron, which most often falls on the surface when using rollers, guillotines, etc., which are made of carbon steel. If you do not remove foreign inclusions and iron particles from the surface of stainless steel, then this will certainly lead to corrosion. After pickling, stainless steel acquires a matte, uniform surface.
| Before | After |
Metal passivation technology, types and compositions
Passivation is the formation of thin oxide or salt films on the metal surface that protect it from external corrosion. This coating prevents metal from coming into contact with oxygen and aggressive environments. During passivation, protective films can form on a metal surface both naturally and artificially. In the first case, they consist of oxides of chemical elements that are part of the metal itself, and in the second, they may include oxides and salts of other chemical elements. For example, pure aluminum naturally forms a very durable oxide film and is therefore resistant to most types of corrosion. But products made from its alloys, containing chemically active components, already require artificial corrosion protection and therefore undergo passivation in saline solutions.
Passivation is widely used to protect the surfaces of products made of steel, copper, nickel, aluminum and their alloys. Even protective zinc and cadmium coatings are passivated with chromium salts to increase their corrosion and mechanical resistance. Passivation of a metal causes the formation of a layer of oxides or salts several microns thick on its surface, which practically does not affect the geometric dimensions of the products. On the other hand, such films can reduce the contact conductivity of the base material, but, as a rule, to a lesser extent than a layer of corroded metal.
Sequence of passivation
The following procedure for carrying out the technology under consideration is recommended:
- Preliminary cleaning of the surface of the part to be passivated from any contamination.
- Chemical treatment by immersing the material in a bath of citric acid.
- Washing in water.
- Neutralization of acid residues in an aqueous solution of sodium carbonate.
- Drying.
- Testing the finished surface (the electrical contact measurement method is used, since the conductivity of the passivated layer is worse than that of a conventional one).
Passivation is recommended for all grades of stainless steel that contain more than 0.02% sulfur (even if the surface visually appears clean and shiny). Particularly desirable is the processing of steels containing sulfides, as well as titanium and tantalum - metals whose oxides are relatively quickly destroyed in a humid atmosphere.
To enhance the efficiency of passivation, sodium dichromate is usually added to acid bath solutions. Options with the simultaneous application of ultrasonic vibrations are more productive: under such conditions, the formation of chromium oxide is intensified, which begins even when the material being processed is in an acid bath.
The thickness of the passivating film is very small - up to 5 microns, but this is enough to reliably protect the stainless steel surface from corrosion.
The essence and description of the metal passivation process
When passivating, the surfaces of metal products are treated with solutions of chemical compounds that have oxidizing properties. This role is most often played by acids, nitrites and solutions of chromium salts (less commonly, molybdenum). The solution is applied to the surface of metal workpieces by immersion or manually using special equipment. Solutions used for passivation usually consist of a main reagent and several additives that accelerate and stabilize the passivation process.
In general, the passivation process consists of the following stages:
- Mechanical cleaning of product surfaces.
- Chemical degreasing in a solution of sodium hydroxide and soda ash.
- Rinse in running hot and then cold water.
- Passivation for a specified time.
- Neutralization in soda ash solution.
- Rinsing by repeated immersion in running cold water.
- Dry in a drying cabinet or blowing warm air.
- Surface quality control after passivation is carried out visually or instrumentally. If the result is unsatisfactory, the passivation process is repeated, starting from step 1.
The given example describes the technological process of passivation using stationary production equipment. To passivate the surfaces of products at the site of their installation, hand-powered tools and devices are used (see photo below).
Etching process
Before etching, it is necessary to thoroughly clean and degrease the metal surface from foreign substances such as grease, oil, adhesives, rust, etc. Surface cleaning can be done with any cleaner, including alkaline cleaners, acidic cleaners, and solvent-based cleaners. The correct cleaning solution is selected based on several factors:
Material and configuration of equipment/parts Level and composition of pollutants
After cleaning and degreasing, the cleaning solution is washed off the surface and etching is carried out using one of the methods mentioned above. Process control is very important as corrosion and pitting can occur if the acid concentration is too high and/or if the acid contact time is too long. Once the process is complete, be sure to ensure that all residual acids are removed and neutralized to prevent pitting and pitting. To achieve optimal corrosion resistance, it is recommended to passivate the stainless steel surface.
Types of passivation
According to the coating application method, passivation is of two types: chemical and electrochemical. In addition, varieties of this technology are classified according to the type of chemical element from the compounds of which the surface film is formed (chromatization, nickel plating, molybdenation, and others). In addition, natural passivation is distinguished - the process of formation of a protective layer in a number of metals and alloys under the influence of atmospheric and dissolved oxygen in water.
Chemical
Chemical passivation occurs as a result of the attraction of negative ions of salts dissolved in water to the surface of the metal, the atoms of which have a positive potential. To do this, metal products, previously cleaned and degreased, are placed in a special bath filled with an appropriate solution. The main component in such an electrolyte is a metal salt that forms a protective film on the surface of the product. Chemical passivation can also be performed at the site where the product is installed. In this case, all processes, from cleaning to passivation, neutralization and washing, are performed manually using special equipment.
Electrochemical
Electrochemical passivation is based on the principles of electroplating. In this case, metal workpieces are also placed in a bath with an electrolyte, but the deposition of the passivation layer does not occur in a passive mode, but under the influence of a current flowing through the electrolytic solution. With this passivation, a positive potential is applied to the workpiece, and a negative potential is applied to the bath body. When using the electrochemical method, the protective film is formed faster and is more even. But this technology is more expensive than chemical passivation, since it uses more complex equipment and consumes electricity.
What is passivation
Passivation - Creation of a thin film of oxides on the surface of metals in order to protect them from corrosion.
The meaning of the word passivation according to Ushakov’s dictionary:
PASSIVATION passivation, pl. no, cf. (from Latin passivus - passive) (tech.). A special treatment of the surface of the metal, as a result of which it becomes incapable of its usual reactions (for example, oxidation) and is likened to noble metals.
Definition of the word “passivation” according to TSB:
Passivation is the passivation of metals, the transition of the metal surface to a passive state, in which corrosion sharply slows down. P. is caused by surface oxidation of metals. The practical significance of P. is extremely great, since all structural metals without their spontaneous P. would be subject to rapid corrosion not only in aggressive chemical environments, but also in the humid earth’s atmosphere or fresh water. If you immerse metal prone to P.
, into a non-oxidizing aqueous electrolyte solution, connect it to a current source that allows you to set any potential values (the so-called potentiostat) and record the dependence of the current density of metal dissolution on the set potential, you will get a polarization curve close to that shown in the figure. The curve shows that the transition of the metal begins at the passivation potential En and the critical current density ip.
With an increase in potential from Ep to Epp (full passivation potential), the current density does not increase, but decreases as a result of P., sometimes by 104-105 times (up to ipp) and then remains almost unchanged up to the repassivation potential Eper. The subsequent new acceleration of dissolution observed is associated with repassivation, or transpassive state. The interval from Epp to Eper is called the region of the passive state. In the presence of Cl&minus., Br&minus., I&minus. ions.
local strong dissolution (“pitting”) of some passive metals begins even at the potential Epit. All of the above quantities are important characteristics of the behavior of metals during corrosion under the influence of oxidizing agents. Thus, a metal correlates with a minimum speed (equivalent to the current density in a completely passive state ipp) when the Redox potential of the medium Eo-v satisfies the condition Epp
was spontaneous (in the absence of external current sources), the rate of reduction of the oxidizer at Ep should be no less than ip. For example, dilute solutions of nitric acid satisfy both of these conditions for chromium, but only the first for iron. Accordingly, Cr in them is passivated itself, and Fe can only maintain a passive state created in some way earlier.
Since for Cr ip and ip are hundreds of times less than for Fe, and Epp and Eper are 0.4-0.5 V more negative, Cr is incomparably more stable than Fe in weakly oxidizing environments, but due to repassivation it is much more destroyed in strong oxidizing agents ( fuming nitric acid, acids with additives of permanganates, chromates, etc.). A strong increase in the concentration of acid or alkali usually leads to an increase in ip and ip, and only some metals are stable in such environments. Among them, Cr, Ni and alloys rich in them, Ti, Zr, are of greatest importance.
In neutral environments, most metals are prone to P. to one degree or another. In non-aqueous solutions, P. is often possible only in the presence of moisture. In P.'s theory, an important role is assigned to both the adsorption of oxygen and the formation of oxide layers. Repassivation is caused by the formation of higher oxygen compounds of the metal, which either dissolve completely, giving anions (CrO42-), or release their cations into the solution, decomposing with the release of oxygen (NiO2).
Some oxidizing agents (H2O2, HNO3) can be sources of oxygen involved in the formation of passivating layers. P. can be promoted by anions that produce sparingly soluble salts or mixed oxides with the metal. However, the most universal source of passivating oxygen is water that reacts chemically or electrochemically with the metal. In technology, the term "P." also means a special chemical or electrochemical treatment of a metal in a suitable solvent, increasing the resistance of its original passive state (P.
aluminum cookware in 30% HNO3, zinc coatings in chromate solutions, etc.). Substances, mainly oxidizing agents, with the help of which P. is produced are called passivators. Lit.: Tomashov N.D., Chernova G.P., Passivity and protection of metals from corrosion, M., 1965. Scorcelletti V.V., Theoretical foundations of metal corrosion, L., 1973. Novakovsky V.M., Justification and the initial elements of the electrochemical theory of dissolution of oxides and passive metals, in the collection: Corrosion and corrosion protection, vol. 2, M., 1973.V. M. Novakovsky.
Rice. to Art. Passivation.
Source: https://xn—-7sbbh7akdldfh0ai3n.xn--p1ai/passivirovanie.html
Contents of passivation compositions
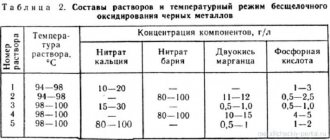
The composition of solutions for passivation of non-ferrous metals most often includes potassium and sodium chromates, as well as chromic anhydride as the main reagent. To create an acidic environment, various acids and salts are added to such electrolytes, the composition of which affects the speed of creation and uniformity of the protective film. Copper passivation is carried out in solutions containing small amounts of sulfuric acid. When processing aluminum, phosphoric acid is included in the electrolytes, and additives in the form of nitric and sulfuric acids are used to passivate zinc and cadmium. The content of passivating solutions for processing steel products depends on their composition and often includes nitric acid and its salts.
All chromium salts (especially hexavalent) are very toxic. Therefore, chrome passivation of metal products can only be carried out in specialized industries that have appropriate cleaning and drainage systems, as well as specially trained personnel.
Nowhere is it written how passivation with chromium salts is carried out directly at the installation sites of the equipment. How are chemicals removed in these cases? Or are other compounds used for this treatment? If anyone has information on this issue, please share it in the comments to our article.