Manganese steels
Manganese is one of the elements that expand the solid solution region and reduce the temperature of the critical point of steel. Manganese refines the structure of steel during secondary crystallization, due to which its plastic properties increase, has little effect on the plastic properties of steel during deformation, reduces the temperature of phase transformations and reduces the rate of formation of carbides from austenite, which improves its hardenability. The formation of complex manganese and iron carbides has a significant impact on the mechanical properties of steel.
Professor of the St. Petersburg Mining Institute V.N. Lipin in 1886 began to study the effect of manganese on the properties of steel. He investigated the effect of manganese on the properties of steel at its content up to 2.5%. Three years earlier, the influence of manganese on the properties of steel with its content up to 20% began to be studied by the English researcher Hadfield. He discovered that steel containing 12% Mn had properties that were not known in other steels at that time. Steel containing 1 - 1.4% C and 10 - 14% Mn is the most common manganese austenitic steel, known as Hadfield steel. Since then, a large number of its modifications have been patented, of which a relatively small number have been mastered by industry.
Currently, cast austenitic manganese steel is widely used in industry; With its low cost and fairly simple production, this steel has high toughness, high resistance to strong impact loads, pressure and good cold hardening, due to which it has high wear resistance. Exceptionally good results are obtained when using this steel for various parts of crushers, excavators and other machines, as well as for armor plates, hammers, strikers, etc. instead of ordinary steel, which wears out quickly under these conditions.
After heat treatment, manganese steel becomes non-magnetic. Since austenitic manganese steels are difficult to machine, they are most often used for the manufacture of castings.
Similar articles
- Austenite decomposition and properties of manganese steel Although austenite in Hadfield steel is very stable (provided the cooling rate is sufficient), in some cases, especially in large castings, […]
- Characteristics of manganese steel The specific gravity of steel with a content of 8 - 10% MP increases compared to the usual by about 1.6%. The specific gravity of manganese steel in a non-magnetic state is always greater than the specific gravity of that […]
- Heat treatment of manganese steel Let's talk about heat treatment of manganese steel. The hardening temperature depends on the carbon content of the steel. The higher the carbon content in steel, the higher […]
- Production of manganese steel For the production of austenitic manganese steels, furnaces with a basic lining are used exclusively. Smelting of manganese steel in acid furnaces is not carried out due to the rapid […]
- Centrifuge design A centrifuge is a horizontal or vertical type installation for dewatering or thickening various types of sewage sludge. The centrifuge device includes: a conical [...]
Domestic low-alloy steels of increased and high strength
Taking into account the conditions of manufacture and operation of structures, as well as economic considerations, a preferable attitude of various branches of the construction complex to one or another grade of steel has been developed. Thus, manufacturers of building structures mainly use steel grades 09G2S and 12G2S (S345 GOST 27772-88) with zero or minus deviation in carbon content in the finished rolled products; in bridge construction, steel grades 15KhSND, 10KhSND, 14G2AFD with increased resistance to atmospheric corrosion are mainly used; in carriage building - steel 09G2D; in boiler making - steel 16GS; for large diameter pipelines - steel 17GS, 17G1S and type 08-12G2FB. Below are the main properties of typical domestic low-alloy steels of the ferrite-pearlite class, widely used in various industries and construction. The results of research carried out by the authors are mainly presented. Manganese Steels Manganese is the traditional and most widely used element in low alloy steels. The widespread use of manganese-containing low-alloy steels is explained by the very favorable influence of manganese on a number of properties of steel. Manganese steels are almost the only type of single alloy steel. Steel grade 09G2 is one of the most common. Created initially as a steel for shipbuilding (good weldability, high ductility, toughness, etc.), it has found wide application in a number of other industries, primarily in carriage building. 09G2 steel is used to produce sheet and profile products in a wide range of thicknesses with a yield strength σТ > 300 N/mm2. Steel 09G2 is smelted both in open hearth furnaces of various tonnages and in converters. The low carbon content with a high manganese content makes it necessary to use mainly silicomanganese when alloying steel, introducing it into the ladle. The most common method of deoxidation and alloying of this steel consists of preliminary deoxidation of the metal in a blast furnace with ferromanganese (6-8 kg/t) and introducing the calculated amount of silicomanganese (22-25 kg/t) into the ladle under a stream. The steel in the ladle is deoxidized with aluminum (0.7-0.8 kg/t) and ferrotitanium at the rate of 0.04% Ti added to the metal (excluding waste). Along with this, the practice of individual plants has established that steel type 09G2 can be produced without preliminary deoxidation of the metal in a furnace, introducing all the ferroalloys into the ladle. When a relatively small amount of ferromanganese is introduced into the furnace, the bath boils vigorously, and all the carbon contained in the ferromanganese is oxidized during the period of deoxidation and release of the melt. At the same time, the temperature of the metal increases rapidly. This additive also has a beneficial effect on the degree of metal desulfurization. The properties of sheet steel 09G2 are significantly influenced by the thickness of the sheets, with an increase in which all indicators of mechanical properties decrease. Hot-rolled sheets with a thickness of over 15 mm, according to the work, had significant sorting by mechanical properties. To increase the level of mechanical properties of sheets with a thickness of 15-20 mm, it is necessary to resort to normalization. Normalization (930° C, heating rate 2 min/mm, cooling on a roller table) made it possible to significantly increase the mechanical properties of such sheets due to grain refinement and greater uniformity of the structure. Comparative studies of steels 09G2 (sheet 11 mm) and 14G2 (sheet 12 mm) were carried out. Hardening followed by tempering of 09G2 steel makes it possible to significantly improve the characteristics and, at the same time, significantly increase impact toughness. Steel 09G2 is relatively insensitive to stress concentration and mechanical aging. Normalization has a positive effect on the impact strength of 09G2 steel after strain aging. A study of the influence of normalization temperature on the cold resistance and mechanical properties of 09G2 steel showed that the maximum values of KCU+2° are obtained at a normalization temperature of 925-950° C. One of the main advantages of 09G2 steel is its good weldability. The low carbon content ensures that cracks in the welding heat affected zone are less likely to occur. Welded joints of steel 09G2, made by automatic welding, are characterized by high impact toughness. The microstructure of 09G2 steel in the hot rolled state consists of ferrite and thin lamellar pearlite. The actual grain size is estimated at 6-8. Currently, the volume of use of 09G2 steel is declining due to its relatively low strength and scarcity of manganese. Steel 14G2 is characterized by a higher carbon content with a lower manganese content than steel 09G2. This determines its better manufacturability during smelting - the ability to begin deoxidation at a slightly higher carbon content and use blast furnace ferromanganese along with silicomanganese. As a rule, preliminary deoxidation and alloying of 14G2 steel with manganese is carried out in a furnace with ferromanganese and silicomanganese in approximately a 1:1 ratio. Additional deoxidation of steel in a ladle is carried out with aluminum (0.5-0.6 kg/t) and ferrogitanium (0.025-0.03% Ti). The strength level of 14G2 steel is higher than that of 09G2 steel (by 30-40 N/mm2), with slightly lower ductility and toughness. Steel 14G2 is a well-welded steel. It is one of the cheapest and easiest to produce low-alloy steels. With increasing thickness of the sheet and especially the universal strip, the strength and ductility characteristics significantly decrease. In this regard, the content of individual elements is strictly regulated depending on the thickness of the rolled product; Technological measures are also taken (for example, strip blowing, additional deoxidation of steel with titanium, silicocalcium, etc.). The effect of thickness on impact strength is to a slightly lesser extent. Normalized sheets of steel 14G2 have high ductility and toughness, including after mechanical aging. In the work, the tendency of steel to brittle fracture was determined by the type of static bending diagrams of samples with a semicircular notch. Of the steels studied in the work, 09G2 steel has the greatest resistance to crack development and brittle fracture in the temperature range from +20 to -70° C, and 14G2 steel (with 0.20% C) has the least resistance. Thermal improvement of 14G2 steel makes it possible to significantly increase the strength characteristics of this steel. Steel after improvement also has a reduced tendency to cold brittleness and less sensitivity to aging. While studying the weldability of 14G2 steel, it was found that it is sensitive to the thermal cycle of welding. However, by appropriate selection of the welding mode, the properties of the base metal in the heat-affected zone can be significantly improved. Welded joints have good deformation ability and practically ensure equal strength of the weld and the base metal. Welds on steel 14G2, as well as on steel 09G2, are highly resistant to the formation of crystallization cracks. Steel 14G2 according to all main indicators should be classified as satisfactorily weldable. A necessary condition for good weldability of 14G2 steel is the correct method of its deoxidation. As indicated in the work, in terms of the main indicators of weldability, steel 14G2 is equivalent to steel 15HSND. The best combination of properties of welded joints of 14G2 steel is ensured when using E50A type electrodes. Due to the relatively low strength and scarcity of manganese, 14G2 steel is currently used to a limited extent.
Manganese steel (110g13l) is now widely used in industry.
Its advantages:
- Low cost.
- Simple production.
- High viscosity.
- High resistance to strong shock loads.
- High pressure resistance.
- High wear resistance.
Steel 110g13l is used in the production of parts for crushers, grinding equipment, excavators and other machines, as well as for armor plates, hammers, strikers, linings, crushing plates, blows, etc., instead of ordinary steel, which wears out quickly under these conditions.
Temirtau Foundry produces:
- Armor and lining made of steel 110G13L.
- The beater and beater holders are made of steel 110G13L.
- Crushing plates made of 110G13L.
| Armor | beat | Crushing plates |
| Bicycle holders | Steel castings | Linings |
- Steel 110G13L
| Classification: | Alloyed casting steel with special properties |
| Application: | housings of vortex and ball mills, cheeks and cones of crushers, teeth and front walls of excavator buckets, railway crosses and other heavily loaded parts operating under static and high dynamic loads and from which high wear resistance is required. Austenitic steel. Steel has a high resistance to wear when simultaneously exposed to high pressures or shock loads. |
| Foreign analogues: | Known |
Chemical composition in % of material 110G13L GOST 977 - 88, also included in GOST 21357-87
| C | Si | Mn | Ni | S | P | Cr |
| 0.9 — 1.5 | 0.3 — 1 | 11.5 — 15 | up to 1 | up to 0.05 | up to 0.12 | up to 1 |
| Note: Material 110G13L is also included in GOST 21357-87, where it has a different chemical. compound |
Technological properties of material 110G13L
| Weldability: | not applicable to welded structures. |
| Flock Sensitivity: | not sensitive. |
| Tendency to temper brittleness: | not inclined. |
Foundry and technological properties of material 110G13L
| Linear shrinkage, %: | 2.6 — 2.7 |
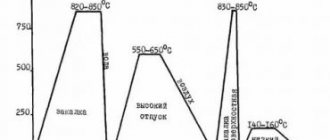
Heat treatment modes for material 110G13L
| Quenching 1050 - 1100 ° C, cooling in water |
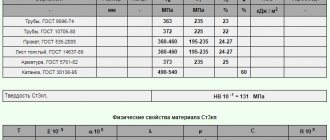
Mechanical properties at T=20oC of material 110G13L
| Assortment | Size | Eg. | sв | sT | d5 | y | KCU | Thermal change |
| — | mm | — | MPa | MPa | % | % | kJ/m2 | — |
| Castings | max thickness 30 | 654-830 | 360-380 | 44 | 37 | |||
| Mechanical properties are established in agreement with the customer | ||||||||
Foreign analogues of material 110G13L Attention! Both exact and closest analogues are indicated.
| USA | Germany | Japan | France | Italy | Sweden | ||||||||||||||||||||||||
| — | DIN,WNr | JIS | AFNOR | UNI | SS | ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
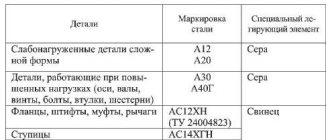
The influence of chemical elements on the properties of steel.
Symbols of chemical elements:
| chromium (Cr) - X nickel (Ni) - H molybdenum (Mo) - M titanium (Ti) - T copper (Cu) - D vanadium (V) - F tungsten (W) - B | nitrogen (N) - A aluminum (Al) - Yu beryllium (Be) - L boron (B) - P bismuth (Bi) - Vi gallium (Ga) - Gl | iridium (Ir) - And cadmium (Cd) - Kd cobalt (Co) - K silicon (Si) - C magnesium (Mg) - Ш manganese (Mn) - G | lead (Pb) - AC niobium (Nb) - B selenium (Se) - E carbon (C) - U phosphorus (P) - P zirconium (Zr) - C |
INFLUENCE OF IMPURITIES ON STEEL AND ITS PROPERTIES
Carbon - found in steel usually in the form of a chemical compound Fe3C called cementite. With an increase in carbon content to 1.2%, the hardness, strength and elasticity of steel increase, but ductility and impact resistance decrease, and workability deteriorates, and weldability also deteriorates.
Silicon - if it is contained in steel in small quantities, it does not have a special effect on its properties. (Useful impurity; introduced as an active deoxidizer and remains in the steel in an amount of 0.4%)
Manganese , like silicon, is found in ordinary carbon steel in small quantities and does not have any particular effect on its properties. (A useful impurity; introduced into steel for deoxidation and remains in it in an amount of 0.3-0.8%. Manganese reduces the harmful effects of oxygen and sulfur.
Sulfur is a harmful impurity. It is found in steel mainly in the form of FeS. This compound makes the steel brittle at high temperatures, such as during forging, a property called red brittleness. Sulfur increases the abrasion of steel, reduces fatigue resistance and reduces corrosion resistance. In carbon steel, no more than 0.06-0.07% sulfur is allowed. (Steel is protected from red brittleness by manganese, which binds sulfur into MnS sulfides).
Phosphorus is also a harmful impurity. Reduces viscosity at low temperatures, that is, it causes cold brittleness. Phosphorus somewhat improves the machinability of steel, as it promotes chip separation.
ALLOYING ELEMENTS AND THEIR INFLUENCE ON STEEL PROPERTIES
Chromium (X) is the cheapest and most common element. It increases hardness and strength, slightly reducing ductility, and increases corrosion resistance; the content of large amounts of chromium makes the steel stainless and ensures resistance to magnetic forces
.
Nickel (N) - imparts corrosion resistance
, high strength and ductility,
increases hardenability
, affects the change in the coefficient of thermal expansion. Nickel is an expensive metal; they are trying to replace it with a cheaper one.
Tungsten (B) - forms very hard chemical compounds in steel - carbides, which sharply increase hardness and red-hardness. Tungsten prevents grain growth when heated and helps eliminate brittleness during tempering. This is an expensive and scarce metal.
Vanadium (F) - increases hardness and strength, grinds grain. It increases the density of steel, as it is a good deoxidizer; it is expensive and scarce.
Silicon (C) - in quantities above 1% has a special effect on the properties of steel: a content of 1-1.5% Si increases strength, while the toughness is maintained. With a higher silicon content, electrical resistance and magnetic permeability increase. Silicon also increases elasticity, acid resistance, and scale resistance.
Manganese (G) - with a content above 1% increases hardness
, wear resistance, resistance to shock loads, without reducing ductility.
Cobalt (K) - increases heat resistance, magnetic properties, increases impact resistance.
Molybdenum (M) - increases red hardness, elasticity, tensile strength, anti-corrosion properties and resistance to oxidation at high temperatures
.
Titanium (T) - increases the strength and density of steel
, promotes grain refinement, is a good deoxidizer, improves workability and corrosion resistance.
Niobium (B) - improves acid resistance and helps reduce corrosion in welded structures.
Aluminum (U) - increases heat resistance and scale resistance.
Copper (D) - increases anti-corrosion properties; it is introduced mainly into construction steel.
Cerium - increases strength and especially ductility.
Zirconium (Z) - has a special effect on the size and growth of grain in steel, refines the grain and makes it possible to obtain steel with a predetermined grain size.
Lanthanum, cesium, neodymium - reduce porosity, help reduce the sulfur content in steel, improve surface quality, and refine grain.
Manganese steels
Manganese as an alloying element is widely used in powder metallurgy. Just like nickel, it belongs to the transition metals. Manganese expands the range of existence of y-Fe, significantly increases the hardness of ferrite, increases the stability of supercooled austenite and reduces the temperature of martensitic transformation. Manganese significantly increases the hardenability of powder steels. It is a carbide-forming element. With carbon it forms Mn3C carbide, which is more stable and durable than iron carbide (cementite). When manganese is introduced into iron-carbon alloys, pure manganese carbides are not formed, but complex (double) carbides of the cementite type (Fe, Mn)3C are always obtained, in which some of the iron atoms are replaced by manganese atoms. Its content in cementite is determined by its amount in steel. In high-manganese steel of the austenitic class, such a double carbide contains more manganese than iron (about 80% Mn and 20% Fe), and in medium-manganese steel with a content of less than 3% Mn, on the contrary, such a carbide contains more iron than manganese (about 80 % Fe and 20% Mn).
The late 70s and early 80s were characterized by growing interest in powdered manganese steels, driven by the need to develop inexpensive alloyed powder steels for mass production. However, the use of manganese (as well as chromium) as an alloying element to produce powder steels is associated with a number of difficulties due to the high affinity of these elements for oxygen.
To reduce the degree of oxidation of manganese and the formation of difficult-to-reduce oxides during the sintering process, it is recommended to use pure starting components and dried sintering media. In addition, it is proposed to introduce HCl, HBr, HF into the sintering medium or introduce boric acid or metal borates into the charge, and use getter fills containing ferroaluminum or ferrosilicon. Manganese can be added to iron powder in the form of crushed ferromanganese or a special alloy. On the contrary, the authors of the work, studying the process of producing manganese steels from a mixture of powders, come to the conclusion that the decisive process should be considered the sublimation of manganese and the formation of a gas phase during sintering. Manganese vapor, settling on iron particles, activates the diffusion of the alloying element. For the most effective effect of sublimation on the alloying and sintering process, in the opinion of the author, manganese should be added in the highest concentration. Under such conditions, manganese vapor released from the compact interacts with oxygen in the protective environment, and the resulting oxides are carried away by the flow and are not formed in the volume of the material.
A number of authors note a decrease in the amount of manganese in the workpiece during the sintering process due to its evaporation. In this case, the loss of the alloying component depends on the proportion of open porosity. An increase in pressing pressure helps to suppress the process of evaporation and entrainment of manganese.
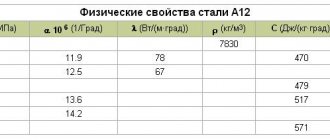
The processes of sintering and structure formation of manganese steels were studied in the work. Powders of reduced and electrolytic iron, ferromanganese with 78% manganese, and graphite were used as starting materials. Sintering was carried out in vacuum at a temperature of 1100 °C. Shrinkage of steels and mechanical properties after sintering are given in table. 31.
The decrease in shrinkage with increasing manganese content is obviously associated with an increase in porosity. It has been established that pores are located in the centers of extended regions of austenite in manganese formed by fine-plate pearlite. It is noted that the strength of sintered steels in all cases was noticeably lower than the strength of cast and heat-treated steels, which, according to the author, is a consequence of the heterogeneity of the material. This leads to the need to introduce a higher amount of alloying elements than should be based on traditional practice.
The structure formation and properties of manganese steels based on atomized and reduced iron powders were studied in the work. Ferromanganese carbon (75% Mn; 7.7% C) with a particle size of 0.04 mm was used as an alloying additive.
Briefly about manganese steel
Manganese steel was invented by Robert Hadfield in 1882, and despite all the research, time and expense involved, the manganese steel currently produced has the same chemical composition as was originally proposed by Hadfield.
Robert Hadfield found that manganese steel is completely different from all others. He tried to quench the forged sample and found that the steel was not harder, like all steels after quenching, but softer. But this was not the only surprise - the new steel could not be turned or milled. Attempts were made to harden Hadfield steel in various environments, but in vain - it remained soft. When it was cold forged, the areas where the hammer struck became hard, and the greater the degree of deformation, the harder the steel became. When processing with a file, a similar phenomenon was observed. The resistance of the metal under the file increased as the pressure was applied: the stronger the pressure, the greater the resistance.
Due to its high hardness and wear resistance, as well as the ability to withstand and absorb strong impacts without destruction, Hadfield steel quickly gained recognition in the industry: it began to be used for the manufacture of those parts that are constantly exposed to strong impacts during operation and usually quickly fail due to abrasion. In the mining industry, these are parts such as, for example, jaws of crushers, beaters for impact crushers, balls for ball mills, and caterpillar tracks.
Explanation of manganese content
There are numerous publications devoted to the study of the effect of manganese on the wear of crusher parts. The differences between steel grades are described below.
- Mn 12-14 – This manganese steel has traditionally been the standard grade for quarry equipment. It is not inferior to other brands in terms of the possibility of hardening during operation. When processing particularly abrasive materials, the hardened layer, typically about 3 mm deep, can be worn or removed by impact abrasion, leading to rapid wear of the softer, unhardened underlying metal. This grade of steel has an initial hardness of about 200 BHN (Brinell hardness). During operation, the hardness increases to approximately 450 BHN.
- Mn 16-18 is typically 7% more expensive to produce than the Mn 12-14 grade. This steel with a high manganese content has approximately the same characteristics as the previous grade. The initial hardness of this steel is slightly higher and is about 230 BHN. Due to the higher carbon content, this grade of steel is hardened faster, and therefore the effect of abrasive wear upon impact is reduced. But the maximum hardness of this steel grade is about 400 BHN. This brand is considered the most versatile material for any application.
- Mn 22-24 - of the manganese steels considered, this grade has the highest initial hardness of 248 BHN, but does not provide more effective hardening than lower grades. In very rare situations it can harden a little faster than lower grades, but its advantages are disproportionate to the cost, which is 14% higher than the cost of Mn 12-14 manganese steel. This brand's offerings are more of a marketing ploy and do not provide any real benefits.
Effect of carbon on manganese content
There is a direct relationship between the amount of carbon that can remain in the alloy and the manganese content. As the carbon content in the alloy increases, the manganese content must also be increased. This gave rise to the myth that increasing the manganese content in the alloy increases the service life of the linings. In fact, it is the carbon content that determines the service life.
To increase the wear life of linings, it is important to have maximum carbon content.
At a Mn content of 18%, the optimal carbon level is achieved.
To determine the required amount of carbon while maintaining the mechanical properties of the alloy, it is necessary to take into account the thickness of the part. The larger the cross-section of the part, the more difficult it is to retain carbon during hardening.