Humanity has learned to use gaseous substances to support artificial processes and reactions, as a result of which it is possible to obtain other chemical compounds. In addition, various gases are used to obtain certain physical phenomena and properties. Carbon dioxide or CO2 has a large number of qualities that cannot but be used in the chemical industry and everyday life.
Carbon dioxide, formula, molecule, structure, composition, substance:
Carbon dioxide (carbon dioxide, carbon dioxide, carbon dioxide, carbon monoxide (IV), carbonic anhydride) is a colorless gas, almost odorless (in high concentrations with a sour “soda” odor).
Carbon dioxide is a binary chemical compound of carbon and oxygen with the formula CO2.
Chemical formula of carbon dioxide CO2.
The structure of the carbon dioxide molecule, the structural formula of carbon dioxide:
Carbon dioxide is approximately 1.5 times heavier than air. Its density under normal conditions is 1.98 kg/m3, relative to air - 1.524. Therefore, it accumulates in low, unventilated places.
The concentration of carbon dioxide in the air (in atmosphere ) averages 0.046% (by mass) and 0.0314% (by volume).
Carbon dioxide is produced in human organs and tissues and is formed as one of the end products of metabolism. It is transported from tissues through the venous system and then released with exhaled air through the lungs. Thus, the content of carbon dioxide in the blood is high in the venous system, decreases in the capillary network of the lungs, and its content is low in the arterial blood. The air exhaled by a person contains about 4.5% carbon dioxide, which is 60-110 times more than the air inhaled. The human body emits approximately 1 kg of carbon dioxide per day.
Carbon dioxide dissolves in water. In 100 grams of water, 0.3803 grams of CO2 dissolves at 16 °C, 0.3369 grams of CO2 - at 20 °C, 0.2515 grams of CO2 - at 30 °C. Dissolving in water, it forms carbonic acid H2CO3. Also soluble in acetone, benzene, methanol and ethanol.
Thermally stable at temperatures below 1000 °C. At a temperature of 1000 °C, coal is reduced to carbon monoxide (II).
At normal atmospheric pressure, carbon dioxide does not exist in a liquid state, only in a solid or gaseous state. When the temperature increases, solid carbon dioxide does not melt, but transforms (sublimes) directly from the solid to the gaseous state. Solid carbon dioxide is also called dry ice. The appearance of dry ice resembles regular ice, a snow-like mass. During sublimation, dry ice absorbs about 590 kJ/kg (140 kcal/kg) of heat.
Under pressure 35,000 atm. solid carbon dioxide becomes a conductor of electric current.
Liquid carbon dioxide can be produced by increasing pressure. Thus, at a temperature of 20 °C and a pressure above 6 MPa (~60 atm), the gas condenses into a colorless liquid. Under normal conditions (20 °C and 101.3 kPa), the evaporation of 1 kg of liquid carbon dioxide produces 509 liters of carbon dioxide. Carbon dioxide is stored and transported, usually in liquid form
Carbon dioxide is non-flammable, but its atmosphere can support the combustion of active metals, for example, alkali metals and alkaline earth metals - magnesium, calcium, barium.
Carbon dioxide is non-toxic and non-explosive.
The maximum permissible concentration of carbon dioxide in the air of the working area has not been established; when assessing this concentration, one can rely on the standards for coal and ozokerite mines, set within 0.5% (vol.) or 9.2 g/m (see GOST 8050- 85 "Carbon dioxide, gaseous and liquid. Technical specifications").
In terms of the degree of impact on the human body, carbon dioxide belongs to the 4th hazard class according to GOST 12.1.007-76.
At concentrations of more than 5% (92 g/m), carbon dioxide has a harmful effect on human health, since it is one and a half times heavier than air and can accumulate in poorly ventilated areas near the floor and in pits, as well as in the internal volumes of equipment for receiving and storing and transportation of gaseous, liquid and solid carbon dioxide. This reduces the volume fraction of oxygen in the air, which can cause oxygen deficiency and suffocation.
Carbon dioxide is formed during the decay and combustion of organic substances as a result of volcanic activity. Contained in the air and mineral springs, released during the respiration of animals and plants. Artificial sources of carbon dioxide formation are industrial emissions and vehicle exhaust gases.
Carbon dioxide easily transmits radiation in the ultraviolet and visible parts of the spectrum, which comes to the Earth from the Sun and heats it.
At the same time, it absorbs infrared radiation emitted by the Earth and is one of the greenhouse gases, as a result of which it participates in the process of global warming.
Application
In the food industry, carbon dioxide is used as a preservative and is indicated on packaging under the code E290
, and also as a dough leavening agent.
Liquid carbon dioxide (liquid food carbon dioxide) is liquefied carbon dioxide stored under high pressure (
65-70 Atm). Colorless liquid. When liquid carbon dioxide is released from a cylinder into the atmosphere, part of it evaporates, and the other part forms dry ice flakes.
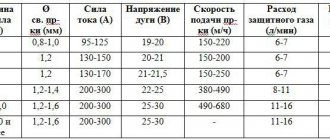
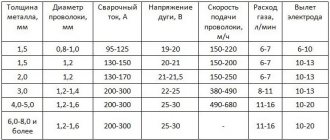
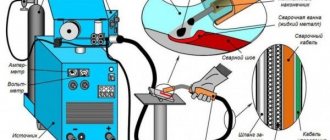
Liquid carbon dioxide cylinders are widely used as fire extinguishers and for the production of carbonated water and lemonade. Carbon dioxide is used as an active medium when welding with wire, since at arc temperature, carbon dioxide decomposes into carbon monoxide CO and oxygen, which in turn interacts with the liquid metal, oxidizing it. Carbon dioxide in cans is used in air guns and as a source of energy for engines in aircraft modeling.
Read also: Grounding in a rural house
Solid carbon dioxide - dry ice - is used as a refrigerant in glaciers and freezing units.
Producing carbon dioxide:
In industry, carbon dioxide is formed in flue gases during the combustion of various organic and inorganic substances or as a by-product of chemical processes, for example, during the decomposition of natural carbonates (dolomite, limestone). Carbon dioxide is also produced as a by-product in air separation plants to produce pure oxygen, nitrogen and argon.
In laboratory conditions, carbon dioxide is obtained, for example, as a result of the following chemical reactions:
1. interaction between calcium carbonate and nitric acid:
CaCO3 + 2HNO3 → Ca(NO3)2 + CO2 + H2O,
2. as a result of the interaction of calcium carbonate with other mineral acids,
3. interaction of baking soda with citric acid or sour lemon juice,
4. carbon combustion reactions:
C + O2 → CO2.
[edit] Application
In the national economy, carbon dioxide is widely used in the chemical industry in the production of soda, urea, etc., as well as in the production of sugar, wine, beer, for the production of carbonated water, etc. Natural sources of carbon dioxide in the form of mineral waters are widely known "Narzan", "Borjomi" and others. Compressed solid CO2 called “dry ice” is used to cool meat, fish and other foods that quickly spoil. Dry ice lowers the temperature much more than regular ice and leaves no liquid behind when it evaporates. In industry, carbon dioxide is produced by the decomposition of calcium carbonate:
Chemical properties of carbon dioxide. Chemical reactions (equations) of carbon dioxide:
Carbon dioxide is an acidic oxide, so it is characterized by the following chemical reactions:
1. reaction between carbon monoxide (IV) and hydrogen:
CO2 + 4H2 → CH4 + 2H2O (t ~ 200 °C, kat = Cu2O).
As a result of the reaction, methane and water are formed.
2. reaction between carbon monoxide (IV) and carbon:
CO2 + C ⇄ 2CO (t = 700-1000 °C).
As a result of the reaction, carbon monoxide (II) is formed. The reaction occurs when carbon dioxide interacts with hot coals.
3. reaction between carbon monoxide (IV) and magnesium:
CO2 + 2Mg → 2MgO + C (t ~ 500 °C).
As a result of the reaction, magnesium oxide and carbon are formed.
4. reaction between carbon monoxide (IV) and hafnium:
Hf + CO2 → HfC + HfO2 (t = 800-1000 °C).
As a result of the reaction, hafnium carbide and hafnium oxide are formed.
5. reaction between carbon (IV) monoxide and germanium:
Ge + CO2 → GeO + CO (t = 700-900 °C).
As a result of the reaction, germanium oxide and carbon (II) monoxide are formed.
6. reaction between carbon monoxide (IV) and zinc:
Zn + CO2 → ZnO + CO (t = 800-950 °C).
As a result of the reaction, zinc oxide and carbon (II) monoxide are formed.
7. reaction between carbon monoxide (IV) and indium:
2In + CO2 → In2O + CO (t ~ 850 °C).
As a result of the reaction, indium oxide and carbon (II) monoxide are formed.
8. reaction between carbon monoxide (IV) and zirconium:
2Zr + CO2 → ZrC + ZrO2 (t = 800-100 °C).
As a result of the reaction, zirconium carbide and zirconium oxide are formed.
9. reaction between carbon monoxide (IV) and tungsten:
W + 2CO2 → WO2 + 2CO (t ~ 1200 °C).
The reaction produces tungsten oxide and carbon (II) monoxide.
10. reaction between carbon monoxide (IV) and lithium oxide:
Li2O + CO2 → Li2CO3.
As a result of the reaction, lithium carbonate is formed.
11. reaction between carbon monoxide (IV) and sodium oxide:
Na2O + CO2 → Na2CO3 (t = 450-550 °C).
The reaction produces sodium carbonate .
12. reaction between carbon monoxide (IV) and potassium oxide:
K2O + CO2 → K2CO3 (t ~ 400 °C).
As a result of the reaction, potassium carbonate is formed.
13. reaction between carbon monoxide (IV) and barium oxide:
BaO + CO2 → BaCO3.
As a result of the reaction, barium carbonate is formed.
14. reaction between carbon monoxide (IV) and calcium oxide:
CaO + CO2 → CaCO3.
As a result of the reaction, calcium carbonate is formed.
15. reaction between calcium carbonate, carbon monoxide (IV) and water:
CaCO3 + CO2 + H2O → Ca(HCO3)2.
As a result of the reaction, calcium bicarbonate is formed.
16. reaction between carbon monoxide (IV) and magnesium oxide:
MgO + CO2 → MgCO3.
The reaction produces magnesium carbonate .
17. reaction between carbon monoxide (IV) and silicon oxide ( II ):
SiO + CO2 → SiO2 + CO (t ~ 500 °C).
As a result of the reaction, silicon (IV) oxide and carbon (II) oxide are formed.
18. reaction between carbon monoxide (IV) and water:
CO2 + H2O ⇄ H2CO3.
As a result of the reaction, carbonic acid is formed.
19. reaction between carbon monoxide (IV) and lithium hydroxide:
2LiOH + CO2 → Li2CO3 + H2O.
The reaction produces lithium carbonate and water. The reaction uses a concentrated solution of lithium hydroxide.
20. reaction between carbon monoxide (IV) and potassium hydroxide:
KOH + CO2 → KHCO3,
2KOH + CO2 → K2CO3 + H2O.
In the first case, the reaction results in the formation of potassium bicarbonate, in the second case - potassium carbonate and water. The reaction takes place in the first case in ethanol and a dilute solution of potassium hydroxide is used, in the second case a concentrated solution of potassium hydroxide is used.
21. reaction between carbon monoxide (IV) and sodium hydroxide:
NaOH + CO2 → NaHCO3,
2NaOH + CO2 → Na2CO3 + H2O.
In the first case, the reaction results in the formation of sodium bicarbonate, in the second - sodium carbonate and water. The first reaction uses a dilute solution of sodium hydroxide, the second uses a concentrated solution of sodium hydroxide.
22. reaction between carbon monoxide (IV) and calcium hydroxide:
Ca(OH)2 + CO2 → CaCO3 + H2O.
The reaction produces calcium carbonate and water.
23. reaction between carbon monoxide (IV) and barium hydroxide:
Ba(OH)2 + CO2 → BaCO3 + H2O.
The reaction produces barium carbonate and water.
24. reaction between carbon monoxide (IV) and methane:
CH4 + CO2 → 2CO + 2H2 (t = 800-900 °C, kat = NiO supported on Al2O3).
As a result of the reaction, carbon monoxide (II) and water are formed.
25. reaction of thermal decomposition of carbon monoxide ( IV ):
2CO2 → 2CO + O2 (t > 2000 °C).
The reaction produces carbon(II) monoxide and oxygen .
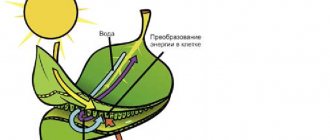
26. photosynthesis reaction:
6CO2 + 6H2O → C6H12O6 + 6O2 (hv, kat = chlorophyll ).
As a result of the reaction, glucose and oxygen are formed.
Applications of carbon dioxide
In everyday practice, carbon dioxide is used quite often. In the food industry, carbon dioxide is used as a leavening agent for dough and also as a preservative. It is indicated on the product packaging under the code E290. The properties of carbon dioxide are also used in the production of sparkling water.
Biochemists have found that to increase the yield of various crops, it is very effective to fertilize the air with carbon dioxide. However, this method of fertilization can only be used in greenhouses. In agriculture, gas is used to create artificial rain. When neutralizing an alkaline environment, carbon dioxide replaces potent mineral acids. In vegetable storage facilities, carbon dioxide is used to create a gaseous environment.
In the perfume industry, carbon dioxide is used in the manufacture of perfumes. In medicine, carbon dioxide is used for antiseptic effects during open operations.
When cooled, carbon dioxide turns into “dry ice.” Liquefied carbon dioxide is packaged in cylinders and sent to consumers. Carbon dioxide in the form of “dry ice” is used to preserve food. When heated, such ice evaporates without leaving a residue.
Carbon dioxide is used as an active medium in wire welding. When welding, carbon dioxide decomposes into oxygen and carbon monoxide. Oxygen interacts with the liquid metal and oxidizes it.
In aircraft modeling, carbon dioxide is used as an energy source for engines. Carbon dioxide canisters are used in air guns.