Heat capacity of materials - table
In construction, a very important characteristic is the heat capacity of building materials. The thermal insulation characteristics of the walls of the building depend on it, and, accordingly, the possibility of a comfortable stay inside the building. Before you begin to familiarize yourself with the thermal insulation characteristics of individual building materials, you need to understand what heat capacity is and how it is determined.
Specific heat capacity of materials
Heat capacity is a physical quantity that describes the ability of a material to accumulate temperature from a heated environment. Quantitatively, specific heat capacity is equal to the amount of energy, measured in J, required to heat a body weighing 1 kg by 1 degree. Below is a table of the specific heat capacity of the most common materials in construction.
In order to calculate the heat capacity of a particular material, you must have the following data:
- type and volume of heated material (V);
- the specific heat capacity of this material (Sud);
- specific gravity (msp);
- initial and final temperatures of the material.
Heat capacity of building materials
The heat capacity of materials, the table for which is given above, depends on the density and thermal conductivity of the material.
And the thermal conductivity coefficient, in turn, depends on the size and closedness of the pores. A fine-porous material, which has a closed pore system, has greater thermal insulation and, accordingly, lower thermal conductivity than a large-porous one.
This is very easy to see using the most common materials in construction as an example. The figure below shows how the thermal conductivity coefficient and the thickness of the material influence the thermal insulation qualities of external fences.
The figure shows that building materials with lower density have a lower thermal conductivity coefficient.
Affiliated (from the English affiliation - connection, connection) - subsidiary; connected, connected.
This word is not often found in colloquial speech, but it can be seen or heard in the news and various analytics, and if we are talking about “insidious oligarchs,” then the matter cannot be done without mentioning certain “affiliated persons.” So, what is it and how is this word and its derivatives correctly spelled?
The word "affiliated" is included in the following expressions:
- affiliated company - a company attached to a larger (parent) company in the form of one of the branches or subsidiaries;
- affiliated persons are individuals and legal entities (in particular, investors) capable of influencing the activities of an economic entity (legal and/or individual, for example, a company) due to participation in its capital or membership in the governing bodies.
Affiliated persons of a legal entity are:
- a member of its board of directors (supervisory board) or other collegial management body, a member of its collegial executive body, as well as a person exercising the powers of its sole executive body;
- persons belonging to the group of persons to which this legal entity belongs;
- persons who have the right to dispose of more than 20 percent of the total number of votes attributable to voting shares or contributions constituting the authorized or share capital, shares of a given legal entity;
- a legal entity in which this legal entity has the right to dispose of more than 20 percent of the total number of votes attributable to voting shares or contributions constituting the authorized or share capital, shares of this legal entity;
- If a legal entity is a participant in a financial-industrial group, its affiliates also include members of boards of directors (supervisory boards) or other collegial management bodies, collegial executive bodies of participants in a financial-industrial group, as well as persons exercising the powers of sole executive bodies of participants in a financial-industrial group. industrial group;
- participants or firms that hide their presence in the activities of a legal entity, but have leverage over it (“protection”, etc.)
Affiliated persons of an individual carrying out entrepreneurial activities (individual entrepreneur) are:
- persons belonging to the group of persons to which the individual belongs;
- a legal entity in which a given individual has the right to dispose of more than 20 percent of the total number of votes attributable to voting shares or contributions constituting the authorized or share capital, shares of this legal entity.
Affiliated site (on the Internet) is an auxiliary site that assists in promoting the main site, a specific company or product, service (satellite site, doorway, or satellite network).
Affiliation is a noun derived from the adjective affiliated.
To remember spelling, let's pay attention to the origin of the word. The English affiliate - to join - comes from the Late Latin filialis - filial (Latin filius - son). From him, by the way, came a branch (an organization that is part of some other organization). Since there is only one “L” in the branch, then in the affiliated (let’s make an analogy) “L” there will also be one. And the double “F” is the result of an English borrowing. It's easy to remember. The commonly used spelling “affiliated” is incorrect.
Sources:
- ru.wikipedia.org - article “Affiliate”;
- newslab.ru - article “New word: affiliated”;
- revolution.allbest.ru - problems with collecting information about affiliated persons;
- mastertalk.ru - topic “Affiliated site... what is it?”
- gramota.ru - the correct spelling and meaning of the word “affiliated”.
Thermal conductivity of non-ferrous metals, heat capacity and density of alloys
The table shows the thermal conductivity values of metals (non-ferrous), as well as the chemical composition of metals and technical alloys in the temperature range from 0 to 600°C.
Non-ferrous metals and alloys: nickel Ni, monel, nichrome; nickel alloys (according to GOST 492-58): cupronickel NM81, NM70, constantan NMMts 58.5-1.54, copel NM 56.5, monel NMZhMts and K-monel, alumel, chromel, manganin NMMts 85-12, invar; magnesium alloys (according to GOST 2856-68), electron, platinum-rhodium; soft solders (according to GOST 1499-70): pure tin, lead, POS-90, POS-40, POS-30, Rose alloy, Wood alloy.
The table shows that magnesium alloys and nickel have high thermal conductivity (at room temperature). Low thermal conductivity is characteristic of nichrome, invar and Wood's alloy.
Thermal conductivity coefficients of aluminum, copper and nickel alloys
The thermal conductivity of metals, aluminum, copper and nickel alloys in the table is given in the temperature range from 0 to 600°C in the dimension W/(m deg). Metals and alloys: aluminum, aluminum alloys, duralumin, brass, copper, monel, nickel silver, nichrome, ferrous nichrome, mild steel. Aluminum alloys have greater thermal conductivity than brass and nickel alloys.
Table of specific heat capacity of some metals and alloys
Go to the updated website >>>
WebVersion of the Reference Book “Thermal Engineering”
MPEI project (V. Ochkov), supported by the Russian Foundation for Basic Research (www.rffi.ru). Diploma of the forum “Educational Environment-2007”.
Description of the resource >>>>>>> Attention! Before opening the calculation, view the figure (pic)
Book 1. Thermal power engineering and heating engineering. General questions (General)
Section 1. In development
Section 2. Units of physical quantities (Units)
Section 3. In development
Section 4. Basic information on mathematics (Mathematic)
Table 4.4. Probability density of normal distribution
Table 4.5. Probability integral
Section 5. Numerical methods, algorithms and software for engineering calculations (Numeric Methods)
Table of specific heat capacity of some metals and alloys
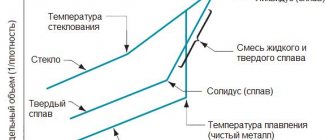
Rice. 15.2. The influence of temperature on the specific modulus of elasticity of various materials
Beryllium is distinguished by high electrical and thermal conductivity, approaching the thermal conductivity of aluminum, and in terms of specific heat capacity [≈ 2500 J/(kg × deg)] it surpasses all other metals. Beryllium is resistant to corrosion. Like aluminum, when beryllium interacts with air, a thin oxide film is formed on its surface, protecting the metal from the action of oxygen even at high temperatures. Only at temperatures above 700 °C do noticeable signs of corrosion appear, and at 1200 °C metallic beryllium burns, turning into white beryllium oxide powder.
Beryllium has high nuclear characteristics - the lowest effective thermal neutron capture cross-section among metals and the highest scattering cross-section.
The disadvantages of beryllium are its high cost, due to the scarcity of raw materials and the complexity of its processing, as well as low cold resistance. The impact strength of technical beryllium is below 5 J/cm 2 .
Despite these disadvantages, the unique combination of technical advantages makes beryllium one of the outstanding aerospace materials.
The main difficulty in alloying beryllium is the small size of its atoms, as a result of which most elements, when dissolved, greatly distort the crystal lattice, imparting increased brittleness to the alloy. Alloying is possible only with those elements that form mechanical mixtures with beryllium with minimal mutual solubility.
The serious disadvantage of beryllium, which is low impact strength and cold brittleness, can be overcome by using alloys with aluminum. From the Al-Be phase diagram it is clear that these elements are practically mutually insoluble (Fig. 15.3). In such eutectic-type alloys, solid beryllium particles are uniformly distributed in a ductile aluminum matrix. The alloys contain 24–43% aluminum, the rest is beryllium. (USA) an alloy containing 62% beryllium, called locelloy, was developed. Be-Al alloys have a structure consisting of soft plastic eutectic and hard brittle inclusions of primary beryllium. These alloys combine the high rigidity, strength and low density characteristic of beryllium with the ductility of aluminum (Fig. 15.4). Due to the plasticity of the matrix, the stress concentration in beryllium phase particles is reduced and the risk of crack formation is reduced, which makes it possible to use alloys under more complex stress conditions.
Powder metallurgy methods are also used to produce beryllium-aluminum alloys. Deformation is carried out by extrusion, followed by forging and stamping in shells. Mechanical properties of pipes made of lokelloy (Be + 38% Al) at room temperature: σв = 600 MPa, σ0.2 = 570 MPa, δ = 1%.
To increase strength, Be-Al alloys are additionally alloyed with magnesium and silver - elements soluble in the aluminum phase. In this case, the matrix is a more durable and tough Al-Mg or Al-Ag alloy.
A plastic matrix can be obtained using a Be–Ag composition containing up to 60% silver. Alloys with silver are additionally alloyed with lithium and lanthanum.
With the exception of alloys with a ductile matrix, alloying with other elements does not eliminate the cold brittleness of beryllium. High purity beryllium has maximum ductility.
Copper alloys with 2–5% beryllium, the so-called beryllium bronzes, are widely used. In Russia, beryllium bronze BrB2 with 2% Be is widely used. From the phase diagram (Fig. 15.5) it is clear that this alloy is dispersion-hardening and can be strengthened by hardening followed by aging. Quenching at 800 °C fixes a supersaturated α-solid solution, from which dispersed CuBe particles are released during aging at 300–350 °C, forming a regular, so-called quasiperiodic structure (Fig. 15.6). After hardening, the properties of beryllium bronze BrB2: σв = 500 MPa, δ = 30%, after aging - σв = 1200 MPa, δ = 4%.
Beryllium bronzes have high elastic properties. They are used to make springs that retain elasticity over a wide temperature range, including cryogenic conditions. They resist fatigue and corrosion well.
Beryllium bronzes are non-magnetic and do not spark upon impact. They are used to make tools for working in explosive environments - mines, gas plants, where ordinary steel cannot be used (Fig. 15.7).
Casting beryllium alloys (LBS), the composition of which is given in table. 15.2, are used for parts of base housings, frames, brackets, etc. Beryllium alloys are characterized by high heat capacity values, which are 1.6 times higher than those of aluminum alloys.
The thermal conductivity and thermal diffusivity of the alloys is only slightly inferior to cast aluminum alloys.
The totality of the thermophysical characteristics of beryllium alloys, in general, distinguishes them favorably from other materials (for example, silumins) and determines high dimensional stability under conditions of temperature gradients during product operation.
The corrosion resistance of beryllium alloys is at a high level. An anodic oxidized film on the surface and paint and varnish coatings additionally provide reliable protection of LBS alloys from corrosion.